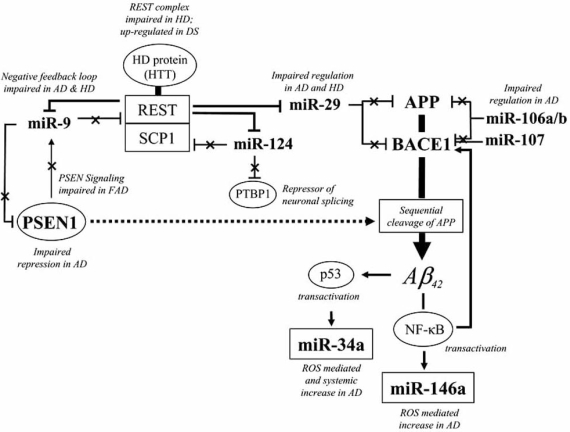
Fig. (2).
A simplified scheme of etiopathogenetic pathways associated with microRNA dyregulation in sporadic Alzheimer disease (AD). Sporadic AD may be characterized by the increase in hallmark genes (i.e. APP, BACE1, PSEN1) which contribute to amyloid beta (Aβ) production by sequential cleavage of APP. Altered miRNAs reported in AD brain which target these genes (as predicted by miRanda and TargetScan algorithms and validated in cell-based assays) are shown [93, 94, 96, 97, 108]. The REST complex negatively regulates the expression of miR-9, miR-29 and miR-124 [134]. The negative feedback loop by miR-9 which suppresses REST is impaired in AD and other neurological diseases. Familial AD (FAD) PSEN1 loss-of-function mutations are recapitulated by PSEN1 knock-out in mouse [72], leading to the decrease in miR-9 akin to that observed in sporadic AD brain. Oxidative stress and DNA damage, generated in part by elevations in amyloid beta (Aβ), iron and reactive oxygen species, induce miRNA expression as exemplified by miR-34a and miR-146a. The stress responsive transcription factor p53, which may increase in AD by Aβ transactivation [173], may further transactivate miR-34a [174]. Stress-mediated neuroinflammatory responses are represented by nuclear factor-kappa B (NF-κB) which transactivates the expression of miR-146a [108] and BACE1 [175]. Barred lines denote post-transcriptional repression of target genes and crossed lines denote de-repression associated with the down-regulation of miRNAs.