Abstract
We hypothesized that maturation-induced vascular inflammation produces endothelial dysfunction in type II diabetes and TNFα plays a key role in triggering inflammation in the development of diabetes. In control (Db/db) mice aged 6, 12, 18 and 24 weeks, sodium nitroprusside (SNP) and acetylcholine (ACh) induced dose-dependent vasodilation, and dilation to ACh was blocked by the NO synthase inhibitor NG-monomethyl-L-arginine. In type II diabetic (db/db) mice at age of 12, 18 and 24 weeks, ACh or flow-induced dilation was blunted compared to Db/db; endothelial function is normal at 6 weeks of age in db/db Vs. control mice, but SNP produced comparable dilation at age of 6, 12, 18 and 24 weeks. Decrements in endothelial function in db/db mice progressively increased from 6–12 to 18–24 weeks. Administration of neutralizing antibodies to TNFα ameliorated endothelial dysfunction in db/db mice aged 12, 18 and 24 weeks. The effect was most prominent in the younger animals. Plasma concentration, expression of TNFα and TNFα receptor 1 (TNFR1) were elevated in coronary arterioles, even at the age of 6 weeks before the development of diabetes in db/db mice compared to control mice. Superoxide production was lower in Db/db mice compared to db/db mice and increments in superoxide production in db/db mice progressively increased from 6–12 to 18–24 weeks. NAD(P)H oxidase inhibitor apocynin attenuated superoxide production in db/db mice at 12 weeks of age, mitochondria respiratory chain inhibitor rotenone attenuated superoxide production at 24 weeks in db/db and Db/db mice, but the combination of apocynin and rotenone reduced superoxide production at 18 weeks for db/db and Db/db mice. The expression of TNFα and its receptors increase progressively with maturation in concert with the development of diabetes. Incremental increases in TNFα/TNFR1 expression induces activation and production of superoxide via NAD(P)H oxidase and/or mitochondria respiratory chain, leading to endothelial dysfunction progressing to the development of type II diabetes.
Keywords: inflammation, cytokines, coronary microcirculation, endothelial dysfunction
Introduction
Cardiovascular disease is the primary mortality and morbidity factor in the United States and aging is a major risk factor for cardiovascular disease. Anti-tumor necrosis factor alpha (TNFα) therapies have proved effective in the treatment of inflammatory diseases such as rheumatoid arthritis and inflammatory bowel disease [3] and elicit modest improvements in cardiac function in patients with heart failure [8] and attenuate the development of experimental diabetic cardiomyopathy [21]. We have reported that TNFα inhibits endothelium-dependent NO-mediated dilation of porcine coronary arterioles via production of O2.− [23]. Flow-induced dilation (FID) is a marker for endothelial function and is measured as the absolute diameter of the vessel (in μm) or as the percentage of contraction induced by 75 mm Hg of pressure and zero flow. A signal transduction cascade of NO, TNFα, and sphingosine is causally involved in the coronary microembolization-induced progressive contractile dysfunction [18]. In coronary microembolization, TNFα is responsible for both progressive contractile dysfunction and delayed protection against infarction [15]. We established the feasibility of a connection between the impaired dilation in a diabetic model (db/db mice) by showing that anti-TNFα restored this impairment, and the NO-mediated vasodilation in db/db null for TNFα (dbTNF−/dbTNF−) mice is normal compared with the vasodilation in Db/db control mice. Based on this information, we investigate several time points at different stages of diabetes, e.g., prediabetes, diabetes, and advanced diabetes, and evaluate coronary endothelial dysfunction to elucidate the role of aging in inducing TNFα production in the development of type II diabetes. These studies allow us to first document the extent of endothelial dysfunction at the different stages of type II diabetes.
The diverse signaling events that regulate varied TNFα responses are all initiated by the binding of heterotrimeric ligand to one of two cell surface receptors (TNFR1 and TNFR2) [20]. The connection between TNFα and type II diabetes-induced endothelial dysfunction was established in the previous study showing anti-TNFα restored endothelium-dependent vasodilation in db/db mice [5]. Schmidt et al. [14] reported that the antiatherogenic/anti-inflammatory effect of lysosphingolipids depends on a competitive interaction of endothelial differentiation gene receptor-induced inhibition and TNFα-initiated stimulation of NFκB translocation into the cell nucleus thereby preventing or stimulating inflammatory events in atherogenesis. The literature is replete with studies supporting the role of inflammation in many vascular pathologies, but none have elucidated the particular cytokine (or cytokines) responsible for the inflammation. We hypothesized maturation exacerbates TNFα induced vascular inflammation, resulting in progressive endothelial dysfunction culminating in type II diabetes. The reasons we currently focus on TNFα are: (1) TNFα is one of the key inflammatory mediators that is expressed during a variety of inflammatory conditions. (2) TNFα initiates the expression of an entire spectrum of inflammatory cytokines ranging from many interleukins to interferons. We evaluated the endothelium-dependent and independent vasodilation, superoxide (O2.−) production, the circulating levels of TNFα and its expression at the wall of coronary arterioles in the development of type II diabetes and explore the mechanisms involved using different ages of diabetic (db/db), lean control (Db/db) mice and db/db mice treated with anti-TNFα.
Methods
Animal models
The procedures followed were in accordance with approved guidelines set by the Laboratory Animal Care Committee at Texas A&M University. Normal control (Db/db, C57BL/6J, stock number is 664) and type II diabetes (db/db, B6.Cg-m +/+ Leprdb/J, stock number is 697) mice were purchased from Jackson Laboratory and maintained on a normal rodent chow diet. Our studies utilized 6, 12, 18 and 24 weeks old, 25–65 g Db/db and db/db mice of either sex.
Measurement of blood parameters
Blood was obtained from vena cava after anesthesia with sodium pentobarbital (50 mg/kg, i.p.), and exposure of the vein. Blood was collected and the plasma was stored at −80°C until analysis.
Plasma concentration of TNFα. TNFα was measured using a commercial kit BIO-Plex cytokine assay (BIO-Plex mouse 3-plex assay, Bio-Rad Laboratories, CA, USA). TNFα concentrations were automatically calculated by BIO-Plex Manager software using a standard curve derived from a recombinant cytokine standard. Values were expressed as pg per ml.
Blood glucose. We used an Accu-check compact glucometer (Roche Diagnostic GmbH, Mannheim, Germany) for measuring blood glucose in Db/db, db/db and db/db mice treated with anti-TNFα with food ad lib at the same time (7:00–8:00AM), every time (Note: absence of a functional leptin receptor in db/db mouse makes food deprivation stressful; this precluded a fasting protocol.)
Lipid level. Serum lipid level was measured by Cholesterol/Cholesteryl Ester Quantitation Kit (Biovision) using spectrophotometry.
Insulin resistance. Blood (~1 ml) was obtained by cardiac puncture with a syringe containing 24 mM EDTA. Insulin resistance was determined by using the Homeostasis Model Assessment (HOMA) that estimates steady state insulin resistance.
Blood pressure (BP). BP was monitored using MacLab/8 data acquisition system (AD Instruments, Milford, MA, USA) equipped with ETH 400 transducer amplifier via femoral artery catheterized with a PE-10 polyethylene tubing.
mRNA expression of TNFα by real-time PCR
Total RNA was extracted from isolated coronary arterioles using Trizol reagent (Life Technologies Inc.), and was processed directly to cDNA synthesis using the SuperScript™ III Reverse Transcriptase (Life Technologies Inc.). The primers of TNFα were designed (primer 3 software) and synthesized (Qiagen) [11]. cDNA was amplified using qRT-PCR Kit with SYBR® Green (Life Technologies Inc.). Data were calculated by 2−ΔΔCT method and presented as fold change of transcripts for TNFα gene in db/db mice normalized to β-actin, compared with Db/db mice (defined as 1.0-fold).
Treatment with TNFα neutralization
The neutralizing antibody to TNFα (anti-TNFα) [10] is 2E2 monoclonal antibody (2E2 MAb. 94021402, NCI Biological Resources Branch). At 6–24 weeks of age, all mice received the neutralizing anti-TNFα (2E2 MAb. 0.625 mg ml−1 kg −1day−1, 3 days, i.p.); dosage was based on our estimates of TNFα expression (in the low ng or pg range) and this is able to neutralize 10–100-fold this amount of TNFα.
Functional assessment of isolated coronary arterioles
The techniques for identification and isolation of coronary microvessels were described in detail previously [9]. Briefly, coronary arterioles (40–100 μm in diameter) from Db/db, db/db and db/db treated with anti-TNFα mice were carefully dissected for in vitro study. The contributions of NO pathway in these vasodilations were examined by treating the vessels with the NOS inhibitor NG-monomethyl-L-arginine (L-NMMA, 10 μmol/l, 30-min incubation).
To determine whether TNFα was playing a role in endothelial injury in type II diabetes, endothelial dependent (ACh, 0.1 nmol/l to 10 μmol/l), independent (sodium nitroprusside, SNP, 0.1 nmol/l to 10 μmol/l) dilation, and flow-induced vasodilation (NO-mediated, endothelial dependent but agonist independent; 4–60 cm H2O) were assessed in coronary arterioles in Db/db, db/db and db/db mice treated with anti-TNFα. Flow is established by the production of a pressure drop across the vessel, and linearly related to the pressure drop (ΔP). All drugs were administered extraluminally in these functional studies.
Measurement of O2.− using electron paramagnetic resonance spectroscopy
O2.− quantitation from the electron paramagnetic resonance (EPR) spectra was determined in the homogenate (4–6 isolated coronary arterioles) at age of 6, 12, 18, and 24 weeks in Db/db and db/db mice as described previously [24]. Briefly, O2.− quantitation from the EPR spectra was determined by double integration of the peaks, with reference to a standard curve generated from horse radish peroxidase generation of the anion from standard solutions of hydrogen peroxide, using p-acetamidophenol as the co-substrate, and then normalized by protein concentration.
Data analysis
At the end of each experiment, the vessel was relaxed with 100-μmol/l SNP to obtain its maximal diameter at 60 cm H2O intraluminal pressure [9]. All diameter changes in response to agonists were normalized to the vasodilation in response to 100 μmol/l SNP and expressed as a percentage of maximal dilation. All data are presented as mean ± SEM. Statistical comparisons of vasomotor responses under various treatments were performed with two-way ANOVA and intergroup differences were tested with Bonferonni Inequality. Significance was accepted at P < 0.05.
Results
Plasma concentrations of glucose/TNFα, body weights, abdominal girth, BP, insulin resistance and lipid level
Glucose concentration, body weight, abdominal girth and lipid level are higher in db/db mice compared with Db/db mice. In db/db mice treated with anti-TNFα, no differences were observed in body weight, abdominal girth, blood glucose or lipid level compared with db/db mice. Higher values of insulin resistance were found in db/db mice and db/db mice treated with anti- TNFα. BP did not differ among the 3 groups on the day of surgery at 6, 12, 18 and 24 weeks. Inflammatory cytokine TNFα progressively increases in db/db mice from 6–12 to 18–24 weeks (Table 1).
Table 1.
Metabolic parameters were measured at 6–24 weeks in different strains of mice (Table 1, n = 10)
| Age | 6 weeks |
12 weeks |
18 weeks |
24 weeks |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Groups | Db/db | db/db | db/db Anti-TNF | Db/db | db/db | db/db Anti-TNF | Db/db | db/db | db/db Anti-TNF | Db/db | db/db | db/db Anti-TNF |
| Body weight (g) | 17 ± 3 | 25 ± 4* | 24 ± 3* | 24 ± 5 | 47 ± 6* | 46 ± 7* | 30 ± 4 | 58 ± 7* | 55 ± 6* | 26 ± 5 | 65 ± 5* | 64 ± 3* |
| Abdominal girth (cm) | 3.6 ± 0.3 | 4.9 ± 0.4 | 4.9 ± 0.2 | 5 ± 0.3 | 15 ± 0.4* | 15 ± 0.3* | 6 ± 0.5 | 17 ± 0.7* | 18 ± 0.9* | 7 ± 0.3 | 23 ± 0.5* | 25 ± 0.8* |
| Glucose (mg/dl) | 122 ± 9 | 167 ± 15* | 162 ± 13* | 139 ± 17 | 283 ± 12* | 299 ± 23* | 149 ± 19 | 344 ± 15* | 327 ± 24* | 144 ± 13 | 367 ± 30* | 359 ± 26* |
| Lipid (μg/ml) | 0.16 ± 0.02 | 0.19 ± 0.03 | 0.17 ± 0.03 | 0.2 ± 0.01 | 0.44 ± 0.02* | 0.40 ± 0.03* | 0.2 ± 0.02 | 0.5 ± 0.03* | 0.5 ± 0.06* | 0.28 ± 0.03 | 0.6 ± 0.02* | 0.63 ± 0.05* |
| TNF (pg/ml) | 1.0 ± 0.01 | 1.7 ± 0.02* | 0.98 ± 0.02 | 1.2 ± 0.03 | 6.89 ± 0.05* | 1.03 ± 0.02 | 1.2 ± 0.03 | 8.6 ± 0.04* | 0.97 ± 0.06 | 1.31 ± 0.02 | 10.6 ± 0.03* | 0.96 ± 0.05 |
| Insulin resistance | 0.0 ± 0.0 | 0.2 ± 0.01 | 0.2 ± 0.02 | 0.0 ± 0.0 | 2.3 ± 0.03* | 2.1 ± 0.02* | 0.0 ± 0.0 | 2.9 ± 0.03* | 2.7 ± 0.04* | 0.1 ± 0.02 | 3.0 ± 0.03* | 2.9 ± 0.03* |
| BP (mm Hg) | 92 ± 6 | 97 ± 7 | 95 ± 5 | 99 ± 5 | 98 ± 5 | 106 ± 5 | 107 ± 5 | 103 ± 5 | 101 ± 5 | 110 ± 9 | 113 ± 11 | 105 ± 5 |
Plasma concentrations of TNFα were also measured using a commercial kit BIO-Plex cytokine assay (BIO-Plex mouse 3-plex assay, Bio-Rad Laboratories, CA, USA) Values are mean ± SE.
P < 0.05 Vs. Db/db mice
mRNA expression of TNFα and TNFRs
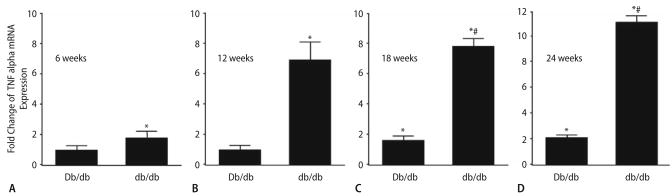
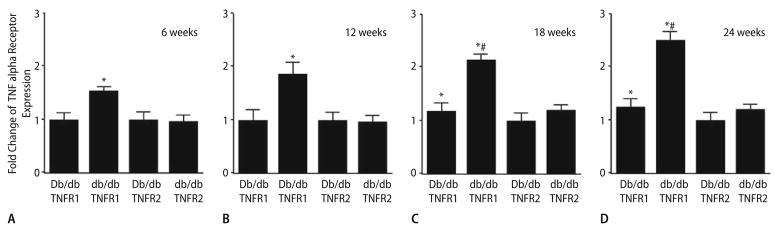
TNFR1 and TNFR2 in isolated coronary arterioles at ages of 6 (A), 12 (B), 18 (C) and 24 (D) weeks for Db/db and db/db mice. The results in Figs. 1 and 2 corroborate the functional results shown in Figs. 3 and 4, in which we found that anti-TNFα prevented endothelial dysfunction in db/db mice aged 12, 18 and 24 weeks.
Fig. 1.
Fold change of TNFα mRNA expression (real-time PCR) is significantly higher in db/db than in Db/db controls aged 6 (A), 12 (B), 18 (C) and 24 (D) weeks and TNFα mRNA expression is also higher in Db/db controls at 18 and 24 weeks Vs. 6 and 12 weeks of Db/db mice. The mRNA expression is the highest in db/db mice aged 24 weeks. In Db/db controls, TNFα significantly increases with age at 18 and 24 weeks. Normalization of TNFα transcripts with β-actin transcripts demonstrated a significantly greater expression of TNFα in the coronary arterioles of db/db Vs. Db/db mice. Data represent mean ± SD, n = 6. * P < 0.05 Vs. Db/db mice (6 and 12 weeks); # P < 0.05 Vs. Db/db mice (18 and 24 weeks)
Fig. 2.
Fold change of TNFR expression (real-time PCR) is significantly higher in db/db than in Db/db controls aged 6 (A), 12 (B), 18 (C) and 24 (D) weeks and TNFR1 mRNA expression is also higher in Db/db controls at 18 and 24 weeks Vs. 6 and 12 weeks in Db/db mice. mRNA expression is the highest in db/db mice aged 24 weeks. In Db/db controls, TNFα significantly increases with age at 18 and 24 weeks. Normalization of TNFR1 and TNFR2 transcripts with β-actin transcripts demonstrated a significantly greater expression of TNFR1 than that of TNFR2 in the coronary arterioles of db/db mice. Data represent mean ± SD, n = 6. * P < 0.05 Vs. Db/db mice (6 and 12 weeks); # P < 0.05 Vs. Db/db mice (18 and 24 weeks)
Fig. 3.
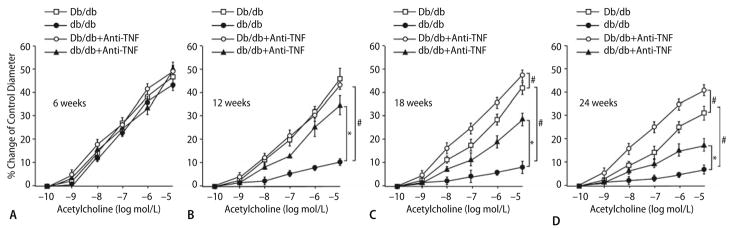
Isolated coronary arterioles from Db/db control mice dilated in a concentration-dependent manner to ACh. ACh-induced vasodilation was blunted in coronary arterioles from db/db mice at 12, 18 and 24 weeks (n = 7, b–d), and ACh-induced vasodilation was also attenuated in coronary arterioles from Db/db mice at 18 and 24 weeks (c, d). However, ACh-induced vasodilation was identical at db/db Vs. Db/db mice at age of 6 weeks (n = 8, a). SNP-induced vasodilation was normal in Db/db, db/db and db/db treated with anti-TNFα (n = 9, data not shown). * P < 0.05 Vs. db/db and # P < 0.05 Vs. Db/db mice
Fig. 4.
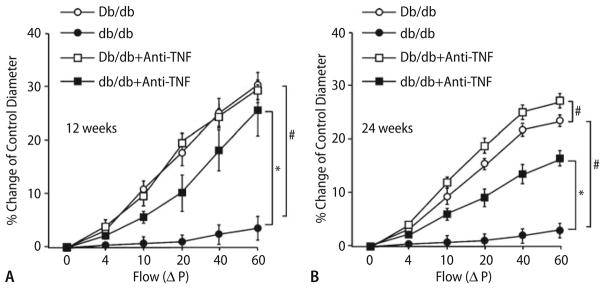
At 12 weeks, anti-TNFα restored NO-mediated coronary arteriolar dilation in db/db mice, but did not affect the vasodilation to ACh in Db/db mice (a, n = 6). a Shows that FID was abrogated in db/db mice (n = 5), but similar to the response for ACh; anti-TNFα restored FID to near control levels (n = 4). Anti-TNFα did not affect dilation of control vessels. At 24 weeks, anti-TNF restored NO-mediated coronary arteriolar dilation in db/db and Db/db mice (b). b Shows that FID in mice of 24 weeks was abrogated in db/db and Db/db mice, and anti-TNFα restored FID at Db/db mice at 24 weeks to the control levels of Db/db mice aged 12 weeks (n = 6), but just partially restored FID at db/db mice aged 24 weeks (n = 4). * P < 0.05 Vs. db/db and # P < 0.05 Vs. Db/db mice
The elevation of TNFα expression is—over 1.7-fold in db/db mice at 6 weeks, over sixfold in db/db mice at 12 weeks, over eightfold in db/db mice at 18 weeks and over 11-fold in db/db mice at 24 weeks Vs. Db/db mice of the same age (Fig. 1). The expression of TNFα in Db/db mice is comparable at 6 and 12 weeks, and increases 1.5-fold at 18 weeks and 1.8-fold by 24 weeks as maturation occurs (Fig. 1).
The elevation of TNFR1 expression is—over 1.5- fold in db/db mice at 6 weeks, over 1.8-fold in db/db mice at 12 weeks, over 2.1-fold in db/db mice at 18 weeks, and over 2.6-fold in db/db mice at 24 weeks Vs. Db/db mice of the same age (Fig. 2), but no difference in TNFR2 expression was found in db/db mice Vs. Db/db mice. Additionally, the elevation of TNFR1 expression is—over 1.3-fold in Db/db mice at 18 weeks, over 1.5-fold in Db/db mice at 24 weeks Vs. the expression of TNFR1 in Db/db mice at 6 and 12 weeks (Fig. 1).
Roles of maturation and TNFα in type II diabetes-induced vascular dysfunction
We previously studied responses to ACh before and after treatment with L-NMMA [5] to show NO dependency of ACh dependent dilation in control mice. Dilation to ACh was significantly attenuated following administration of L-NMMA in control mice [5], which indicates vasodilation to ACh was NO-mediated. At age of 6 weeks (Fig. 3a), endothelial function is normal in db/db and db/db treated with anti-TNFα Vs. Db/db mice. At age of 12 weeks (Fig. 3b), endothelial function is attenuated in db/db Vs. Db/db mice, but anti-TNFα partially restored the vasodilation to ACh in db/db mice without affecting the vasodilation to ACh in Db/db mice. Endothelial function is further and progressively impaired at 18 and 24 weeks of age (Fig. 3c, d) in db/db Vs. Db/db mice, and vasodilation to ACh was also reduced in Db/db mice at 18 and 24 weeks Vs. Db/db mice at 6 and 12 weeks. Anti-TNFα restored vasodilation to ACh in Db/db and db/db mice (Fig. 3c, d).
The concentration–diameter relationships for NO donor SNP (1 nmol/l to 1 μmol/l) were established. SNP-induced endothelial-independent vasodilation was normal in both Db/db and db/db mice at 6, 12, 18 and 24 weeks (data not shown).
Another endothelium dilatory event, FID (Flow is linearly related to the ΔP), a response that is nitric oxide-mediated, endothelial-dependent, but agonist-independent, follows the same trend. FID was abrogated (response shifted rightward) in db/db mice, but similar to the response for ACh at 12 weeks (Fig. 4a) and 24 weeks (Fig. 4b); neutralizing antibodies to TNFα restored FID to near control levels at 12 weeks and partially restored FID to 60% at 24 weeks. Importantly, anti-TNFα did not affect FID of control vessels in Db/db mice at 6 (data not shown) and 12 weeks (Fig. 4a) of age, but prevented vasodilation at 18 (data not shown) and 24 weeks (Fig. 4b) in Db/db mice.
Type II diabetes-induced O2.− production in coronary arterioles in the development of type II diabetes
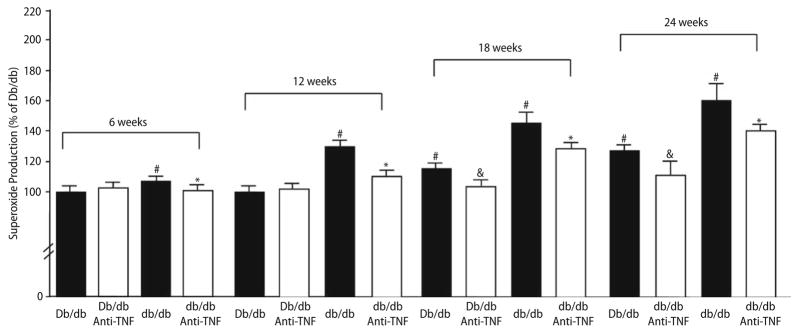
Fig. 5 shows the results from EPR spectroscopy that quantify the production of O2.− and reflect agreement with results obtained by functional study and discussed above. O2.− production was higher in isolated coronary arteries from db/db mice Vs. Db/db mice at 12, 18 and 24 weeks, but O2.− production was normal in isolated coronary arteries from db/db mice Vs. Db/db mice at 6 weeks. Administration of anti-TNFα reduced the production of O2.− to the level observed in the Db/db controls at 12 weeks; anti-TNFα improved O2.− production in db/db mice, but not to the level observed in the Db/db controls at 18 and 24 weeks. Furthermore, anti-TNFα also attenuated O2.− production in Db/db mice at 18 and 24 weeks, but did not affect O2.− production in Db/db mice at 6 and 12 weeks.
Fig. 5.
Results from EPR spectroscopy to quantify the production of O2.− show that O2.− production was higher in isolated coronary arteries from db/db mice Vs. Db/db mice aged 6, 12, 18 and 24 weeks. O2.− production was highest at 24 weeks. In the control group (Db/db mice), O2.− production goes up as well significantly with age at 18 and 24 weeks. Administration of anti-TNFα reduced the production of O2.− to the level observed in the Db/db controls at 12 weeks, but not at 18 and 24 weeks (Fig. 5, n = 9). * P < 0.05 Vs. db/db; # P < 0.05 Vs. Db/db mice; & P < 0.05 Vs. Db/db mice (18 and 24 weeks)
To explore how TNFα produces endothelial dysfunction in diabetes by stimulating production of O2.−, we administered the inhibitors of NAD(P)H oxidase, mitochondrial and xanthine oxidase to the animals and measured O2.− production. NAD(P)H oxidase inhibitor apocynin partially attenuated O2.− production at 12 weeks in db/db mice (Table 2), which is consistent with our previous study [5]. Administration of mitochondria respiratory chain inhibitor rotenone attenuated s O2.− production at 24 weeks in db/db and Db/db mice, but the combination of apocynin and rotenone reduced O2.− production at 18 weeks in db/db and Db/db mice. However, the simultaneous application of allopurinol did not afford more “protection” than that seen with the combination of apocynin and rotenone.
Table 2.
Superoxide production was measured by EPR spectroscopy
| Groups | Db/db | Db/db Apocynin | db/db | db/db Apocynin |
|---|---|---|---|---|
| 6 weeks | 100 ± 4 | 101 ± 3 | 114 ± 6# | 93 ± 4* |
| 12 weeks | 100 ± 3 | 102 ± 5 | 134 ± 7# | 109 ± 6* |
|
| ||||
| Db/db | Db/db Apocynin rotenone | db/db | db/db Apocynin rotenone | |
|
| ||||
| 18 weeks | 116 ± 8# | 97 ± 5& | 151 ± 12# | 110 ± 9* |
|
| ||||
| Db/db | Db/db Rotenone | db/db | db/db Rotenone | |
|
| ||||
| 24 weeks | 127 ± 11# | 109 ± 6& | 158 ± 17# | 116 ± 8* |
O2.− production was higher in isolated coronary arteries from db/db mice Vs. Db/db mice aged 6, 12, 18 and 24 weeks. Administration of NAD(P)H oxidase inhibitor apocynin reduced the production of O2.− in db/db mice at 6 and 12 weeks (n = 9); administration of apocynin and mitochondria respiratory chain inhibitor rotenone reduced the production of O2.− in Db/db and db/db mice at 18 weeks (n = 9); and administration of rotenone reduced the production of O2.− in Db/db and db/db mice at 24 weeks (n = 9)
Values are mean ± SE.
P < 0.05 Vs. Db/db mice;
P < 0.05 Vs. Db/db mice (18 and 24 weeks); and
P < 0.05 Vs. db/db
Discussion
Our results suggest that the inflammatory cytokine TNFα is the key factor in producing O2.− and inducing endothelial dysfunction in db/db (a model for obesity and type II diabetes) and Db/db (lean control) mice as they age. Our findings support the idea that TNFα activates and produces O2.− via NAD(P)H oxidase and/or mitochondrial respiratory chain in endothelial dysfunction in the development of type II diabetes.
Maturation increases mRNA expression of TNFα and TNFRs in type II diabetes
Because of the aging of the population and an increasing prevalence of obesity and sedentary life habits in the United States, the prevalence of diabetes is increasing [7]. Cardiovascular disease is significantly increased in patients with metabolic syndrome and type II diabetes [7]. Factors such as chronic hyperglycemia, lipid abnormalities, inflammation, oxidative stress, endothelium dysfunction, increased thrombosis and decreased fibrinolysis are likely to promote cardiovascular events in patients with metabolic syndrome or type II diabetes [19]. TNFα is a pro-inflammatory cytokine that has been implicated in the pathogenesis of septic, traumatic, and hypovolemic shock-associated cardiac dysfunction, as well as with cardiovascular diseases such as acute myocardial infarction (AMI), chronic heart failure, atherosclerosis, viral myocarditis, and cardiac allograft rejection [12]. Yudkin et al. [22] showed that CRP, IL- 6 and TNFα are elevated in association with quantitative measures of insulin resistance and features of the insulin resistance syndrome. Moreover, the periprocedural release of plaque-derived TNFα possibly represents the amount and activity of the atherosclerotic process and might be a predictor for restenosis [1]. Unstable coronary artery plaques were similarly correlated compared to carotid plaques from symptomatic patients with the accumulation of T cells and the production of T cell chemokines IFNγ and TNFα [4].
Our results demonstrated that plasma concentration, expression of TNFα and TNFR1 were elevated in coronary arterioles, even at the age of 6 weeks before the development of diabetes in db/db mice compared to control mice. Moreover, the expression of TNFα and TNFR1 were progressively increased from the age of 6–12 to 18–24 weeks. Elevation of TNFα occurs at an earlier age in db/db mice than in the control, but, in time, similar elevation occurs in the control, apparently as a normal consequence of maturation. Current evidence [5] indicates that TNFα initiates a cytokine cascade that elevates O2.− levels to increase inflammation in a process that also includes stimulating higher levels of TNFα, ultimately resulting in diabetes. The initiation of the process appears due to TNFα, but as these matters progress, feedback mechanisms come into play where the process itself stimulates higher levels of TNFα.
The role of maturation in impaired vasodilation in type II diabetes
Isolated mesenteric arteries and arterioles from 24 months old F344 rats had reduced shear-mediated dilation response and reduced nitrite perfusate compared to 6-month-old rats [17]. Soucy et al. [16] showed using isolated aortic strips from young (3– 4 months) and old (22–24 months) rats that shear-mediated NO production was significantly attenuated with age. Rask-Madsen et al. [13] have recently demonstrated that TNFα is able to inhibit insulin-stimulated glucose uptake as well as endothelium-dependent vasodilation in humans and that the inhibitory effect on vasodilator function is larger during local elevation of plasma insulin; the authors also speculated that TNFα blocked NO production stimulated by insulin and ACh. TNFα inhibits NO-mediated endothelium-dependent vasorelaxation in small coronary arteries via sphingomyelinase activation and consequent O2.− production in endothelial cells [25]. However, there is no report regarding the role of TNFα in endothelial dysfunction in the development of type II diabetes and the mechanisms by which TNFα induces these pathophysiological conditions. Our results show that decrements in endothelial function and increments of O2.− production in db/db mice progressively increased from 6–12 to 18–24 weeks and endothelial function is normal at 6 weeks in db/db mice. SNP produced comparable endothelial-independent vasodilation in db/db and Db/db mice aged 6, 12, 18 and 24 weeks. Most importantly, administration of neutralizing antibodies to TNFα ameliorated endothelial dysfunction in db/db mice aged 6, 12, 18 and 24 weeks. The effect was most prominent in the younger animals at 12 weeks, which indicate there might be some other pathways involved in advanced type II diabetes-induced endothelial dysfunction and in maturation-induced endothelial dysfunction at 18 and 24 weeks. These functional studies support our hypothesis and correlate the diabetic phenotyping to better understand the basis for endothelial dysfunction. Our functional physiological observations support the present molecular results. It is provocative to note that despite the similarities in glucose, body weights, lipid level, insulin resistance and BP in diabetic animals, endothelial function was better in db/db mice treated with anti-TNFα at 12/18/24 weeks in db/db mice, and 18/24 weeks in Db/db mice. This suggests that TNFα is the key cytokine, that induced endothelial dysfunction in the development of type II diabetes.
The role of maturation in O2.− production in type II diabetes
The potentially important mechanism for the redox control of NO is oxidant stress due to increased levels of ROS. Oxidative stress has been shown to increase with age, and the major molecule involved may be O2.−. Decreased bioavailability of NO could be caused by O2.− scavenging NO or ROS effects on NO production pathways. Our previous studies [5] indicated that O2.− production is greater in db/db mice, and expression of NAD(P)H oxidase are higher in db/db mice. Free radical scavenger, TEMPOL, and NAD(P)H oxidase inhibitor apocynin, partially restored the impaired vasodilation to ACh in db/db mice compared with the Db/db control mice at 12 weeks. Our study shows that anti-TNFα significantly decreased O2.− production in db/db mice aged 12, 18 and 24 weeks, and in Db/db mice aged 18 and 24 weeks. Although previous studies have shown elevated oxidative stress in diabetes, the basis for elevation is still unknown. Moreover, no one has linked TNFα with elevated oxidative stress and endothelial dysfunction in the development of diabetes. A recent study has shown that TNFα inhibits NO-mediated endothelium-dependent vasodilation in small coronary arteries [25]. Our previous study showed that an increase in the circulating and/or local TNFα production in type II diabetes stimulates endothelial generation of O2.− radicals through activation of NAD(P)H oxidase in db/db mice, which contributes to endothelial dysfunction [5]. Our results provide insight into the basis for the endothelial dysfunction induced by oxidative stress in type II diabetes, which indicates that O2.− production is postulated to be linked to TNFα. The present study indicates that the model of type II diabetes increases TNFα, which stimulates endothelial generation of O2.− as endothelium ages and contributes to the endothelial dysfunction. Our results are consistent with these studies and support our hypotheses that TNFα and O2.− are connected in the production of oxidative stress and endothelial dysfunction in the development of type II diabetes. The implication of this scheme is that oxidative stress begets further oxidative stress in the obese diabetic animal, and may explain the development and evolution of vascular pathology progressively degenerating from a healthy condition.
Csiszar et al. [2] showed that aging in many tissues is associated with profound alterations in mitochondrial energy metabolism that likely results in a substantial decline in cellular ATP supply, changes in the NAD/DADH ratio, and/or increased ROS generation. Guzik et al. [6] measured O2.− production in diabetic and non-diabetic vessels in response to a range of potential oxidase inhibitors. Their results show that oxypurinol and rotenone had minimal or modest effect on O2.− production, while diphenylene iodonium, an inhibitor of flavin-containing oxidases such as NAD(P)H oxidases, abolished O2.− production. We previously showed that the production of TNFα is basal to the process of eliciting this oxidative stress [5]. Guzik et al. [6] reported that the protein expression of NAD(P)H oxidase subunits, p22, p47 and p67- phox are significantly increased in diabetic human tissue Vs. normal control. We can state with conviction that the major source of O2.− was NAD(P)H oxidase in type II diabetes since NAD(P)H oxidase inhibitor apocynin significantly reduced O2.− production as measured by EPR. Although there are multiple intracellular sources for formation of oxygen free radicals, our results support the idea that the major enzyme activated by TNFα in type II diabetes is NAD(P)H oxidase at 12 weeks; is mitochondria at 24 weeks; and is a transition from NAD(P)H oxidase to mitochondria during the interim. And, the major enzyme activated by TNFα in control Db/db mice is NAD(P)H oxidase at 18 weeks; and is mitochondria at 24 weeks (Table 2).
In conclusion, we found that TNFα overexpression impairs endothelium-dependent vasodilation in coronary arterioles of type II diabetic mice at 12, 18 and 24 weeks, and the impaired endothelial function can be restored toward normal by administration of TNFα antibodies at 12 weeks in db/db mice and partially restored at 18 and 24 week old db/db mice. The mechanism by which TNFα affects endothelial function is through an increased O2.− production by NAD(P)H oxidases (12–18 weeks), which in turn leads to a reduced NO bioactivity; and the mechanism by which TNFα affects endothelial function is through an increased O2.− production by mitochondria (18– 24 weeks), which in turn leads to a reduced NO bioactivity at 18–24 weeks by direct scavenging. These results confirmed that TNFα plays a pivotal role in the vascular pathology of type II diabetes and provides new findings in the understanding of interactions between TNFα and inflammation, diabetes and atherosclerosis. These findings may provide further insight into a novel therapeutic target for cardiovascular diseases associated with elevated levels of TNFα in the development of type II diabetes.
Acknowledgments
This study was supported by grants from Pfizer Atorvastatin Research Award (2004-37), American Heart Association Scientist Development Grant (110350047A) and NIH grants (RO1-HL077566 and RO1- HL085119) to Dr. Cuihua Zhang.
Footnotes
Conflicts of interest None.
References
- 1.Böse D, Leineweber K, Konorza T, Zahn A, Bröcker-Preuss M, Mann K, Haude M, Erbel R, Heusch G. Release of TNF-alpha during stent implantation into saphenous vein aortocoronary bypass grafts and its relation to plaque extrusion and restenosis. Am J Physiol Heart Circ Physiol. 2007;292:H2295–H2299. doi: 10.1152/ajpheart.01116.2006. [DOI] [PubMed] [Google Scholar]
- 2.Csiszar A, Labinskyy N, Orosz Z, Ungvari Z. Altered mitochondrial energy metabolism many play a role in vascular aging. Med Hypotheses. 2006;67:904–908. doi: 10.1016/j.mehy.2006.03.037. [DOI] [PubMed] [Google Scholar]
- 3.Elliott MJ, Maini RN, Feldman AM. Randomised double-blind comparison of chimeric monoclonal antibody to tumour necrosis factor alpha (cA2) versus placebo in rheumatoid arthritis. Lancet. 1994;344:1105–1110. doi: 10.1016/s0140-6736(94)90628-9. [DOI] [PubMed] [Google Scholar]
- 4.Erbel C, Sato K, Meyer FB, Kopecky SL, Frye RL, Goronzy JJ, Weyand CM. Functional profile of activated dendritic cells in unstable atherosclerotic plaque. Basic Res Cardiol. 2007;102:123–132. doi: 10.1007/s00395-006-0636-x. [DOI] [PubMed] [Google Scholar]
- 5.Gao X, Belmadani S, Picchi A, Xu X, Potte BJ, Tewari-Singh N, Capobianco S, Chilian WM, Zhang C. Tumor necrosis factor alpha-induces endothelial dysfunction in Leprdb mice. Circulation. 2007;115:245–254. doi: 10.1161/CIRCULATIONAHA.106.650671. [DOI] [PubMed] [Google Scholar]
- 6.Guzik TJ, Mussa S, Gastaldi D, Sadowsky J, Ratnatunga C, Pillai R, Channon KM. Mechanisms of increased vascular superoxide production in humans diabetes mellitus. Role of NADPH oxidase and endothelial nitric oxide synthase. Circulation. 2002;105:1656–1662. doi: 10.1161/01.cir.0000012748.58444.08. [DOI] [PubMed] [Google Scholar]
- 7.Haffner SM, Lehto S, Ronnemaa T. Mortality from coronary heart disease in subjects with type 2 diabetes and in non-diabetic subjects with and without prior myocardial infarction. N Engl J Med. 1998;339:229–234. doi: 10.1056/NEJM199807233390404. [DOI] [PubMed] [Google Scholar]
- 8.Kadokami T, Frye CS, Lemster BH. Anti-tumor necrosis factor-alpha antibody limits heart failure in a transgenic model. Circulation. 2001;104:1094–1097. doi: 10.1161/hc3501.096063. [DOI] [PubMed] [Google Scholar]
- 9.Kuo L, Davis MJ, Chilian WM. Myogenic activity in isolated subepicardial and subendocardial coronary arterioles. Am J Physiol. 1988;255:H1558– H1562. doi: 10.1152/ajpheart.1988.255.6.H1558. [DOI] [PubMed] [Google Scholar]
- 10.Lattime ECSO. Thymic lymphomas mediate non-MHC-restricted, TNF-dependent lysis of the murine sarcoma WEHI-164. Cell Immunol. 1991;136:69–79. doi: 10.1016/0008-8749(91)90382-l. [DOI] [PubMed] [Google Scholar]
- 11.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT method. Methods. 1991;25:402– 408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 12.Meldrum DM, Cleveland JC, Cain BS. Increased myocardial tumor necrosis factor-α in a crystalloid-perfused model of cardiac ischemia-reperfusion injury. Ann Thorac Surg. 1998;65:439–443. doi: 10.1016/s0003-4975(97)01297-6. [DOI] [PubMed] [Google Scholar]
- 13.Rask-Madsen C, Dominguez H, Ihlemann N. Tumor-necrosis factor-α inhibits insulin’s stimulating effect on glucose uptake and endothelium-dependent vasodilation in humans. Circ Res. 2003;203:1815–1821. doi: 10.1161/01.CIR.0000091406.72832.11. [DOI] [PubMed] [Google Scholar]
- 14.Schmidt A, Geigenmüller S, Völker W, Buddecke E. The antiatherogenic and anti-inflammatory effect of HDL-associated lysosphingolipids operates via Akt - > NF-kappaB signalling pathways in human vascular endothelial cells. Basic Res Cardiol. 2006;101:109–116. doi: 10.1007/s00395-005-0582-z. [DOI] [PubMed] [Google Scholar]
- 15.Skyschally A, Gres P, Hoffmann S, Haude M, Erbel R, Schulz R, Heusch G. Bidirectional role of tumor necrosis factor-alpha in coronary microembolization: progressive contractile dysfunction versus delayed protection against infarction. Circ Res. 2007;100:140–146. doi: 10.1161/01.RES.0000255031.15793.86. [DOI] [PubMed] [Google Scholar]
- 16.Soucy KG, Ryoo S, Benjo A, Lim HK, Gupta G, Sohi JS, Elser J, Aon MA, Nyhan D, Shoukas AA, Berkowitz DE. Impaired shear stress-induced nitric oxide production through decreased NOS phosphorylation contributes to age-related vascular stiffness. J Appl Physiol. 2006;101:1751–1759. doi: 10.1152/japplphysiol.00138.2006. [DOI] [PubMed] [Google Scholar]
- 17.Sun D, Huang A, Yan EH, Wu Z, Yan C, Kaminski P, Oury TD, Wolin MS, Kaley G. Reduced release of nitric oxide to shear stress in mesenteric arteries of aged rats. Am J Physiol Heart Circ Physiol. 2004;286:H2249–H2256. doi: 10.1152/ajpheart.00854.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thielmann M, Dörge H, Martin C, Belosjorow S, Schwanke U, van De Sand A, Konietzka I, Büchert A, Krüger A, Schulz R, Heusch G. Myocardial dysfunction with coronary microembolization: signal transduction through a sequence of nitric oxide, tumor necrosis factor-alpha, and sphingosine. Circ Res. 2002;90:807–813. doi: 10.1161/01.res.0000014451.75415.36. [DOI] [PubMed] [Google Scholar]
- 19.Verges B. Clinical interest of PPARs ligands. Diabetes Metab. 2004;30:7–12. doi: 10.1016/s1262-3636(07)70083-6. [DOI] [PubMed] [Google Scholar]
- 20.Wallach D, Varfolomeev EE, Malinin NL. Tumor necrosis factor receptor and Fas signaling mechanisms. Annu Rev Immunol. 1999;17:331–367. doi: 10.1146/annurev.immunol.17.1.331. [DOI] [PubMed] [Google Scholar]
- 21.Westermann D, Van Linthout S, Dhayat S, Dhayat N, Schmidt A, Noutsias M, Song XY, Spillmann F, Riad A, Schultheiss HP, Tschöpe C. Tumor necrosis factor-alpha antagonism protects from myocardial inflammation and fibrosis in experimental diabetic cardiomyopathy. Basic Res Cardiol. 2007;102:500–507. doi: 10.1007/s00395-007-0673-0. [DOI] [PubMed] [Google Scholar]
- 22.Yudkin GS, Stehouwer CD, Emeis JJ. C-reactive protein in healthy subjects: associations with obesity, insulin resistance, and endothelial dysfunction: a potential role for cytokines originating from adipose tissue? Arterioscler Thromb Vasc Biol. 1999;19:972– 978. doi: 10.1161/01.atv.19.4.972. [DOI] [PubMed] [Google Scholar]
- 23.Zhang C, Hein TW, Wang W, Ren Y, Shipley RD, Kuo L. Activation of JNK and xanthine oxidase by TNF-α impairs nitric oxide-mediated dilation of coronary arterioles. J Mol Cell Cardiol. 2006;40:47–257. doi: 10.1016/j.yjmcc.2005.11.010. [DOI] [PubMed] [Google Scholar]
- 24.Zhang C, Xu X, Potter BJ, Wang W, Kuo L, Michael L, Bagby GJ, Chilian WM. TNF-alpha contributes to endothelial dysfunction in ischemia/reperfusion injury. Arterioscler Thromb Vasc Biol. 2006;26:475–480. doi: 10.1161/01.ATV.0000201932.32678.7e. [DOI] [PubMed] [Google Scholar]
- 25.Zhang DX, Yi FX, Zou AP. Role of ceramide in TNF-α-induced impairment of endothelium-dependent vasorelation in coronary arteries. Am J Physiol Heart Circ Physiol. 2002;283:H1758– H1794. doi: 10.1152/ajpheart.00318.2002. [DOI] [PubMed] [Google Scholar]