Abstract
An international, multi-lab trial was conducted to evaluate a flow cytometry-based method for scoring micronuclei in mouse lymphoma L5178Y cells [Avlasevich et al., Environ. Molec. Mutagen. 47 (2006) 56–66]. A reference laboratory investigated the potential of six chemicals to induce micronuclei—the genotoxicants mitomycin C, etoposide, and vinblastine, and the non-genotoxicants sucrose, staurosporine, or dexamethasone. The latter two non-genotoxicants were selected as extreme challenges to the assay because of their potent apoptogenic activity. Three collaborating laboratories were supplied with prototype In Vitro MicroFlow™ kits, and each was assigned one genotoxicant and one non-genotoxicant. Cells were treated continuously for 24 hrs over a range of concentrations up to 5 mg/ml, or overtly cytotoxic concentrations. Micronuclei were scored via standard microscopy and flow cytometry. In addition to enumerating micronucleus frequencies, a cytotoxicity measurement that is simultaneously acquired with the flow cytometric micronucleus scoring procedure was evaluated (Flow-NBR). With this method, latex particles served as counting beads, and facilitated relative survival measurements that exclude the presence of dead/dying cells. For comparison purposes, additional cytotoxicity endpoints were measured, including several that are based on cell number, and others that reflect compromised membrane integrity, including dye permeability and/or phospholipid distribution. Key findings for this set of compounds include the following: (1) significant discrepancies in top concentration selection were found when cytotoxicity measurements were based on different methods, with the Flow-NBR approach tending to be the most sensitive, (2) both microscopy- and flow cytometry-based scoring methods detected concentration-dependent micronucleus formation for the three genotoxic agents studied, with good agreement between the reference laboratory and the collaborating laboratories, and (3) whereas flow cytometric analyses showed no significant increases for the non-genotoxicants when top concentration selection was based on Flow-NBR, significantly elevated micronucleus frequencies were observed for concentrations that were chosen based on less-sensitive cytotoxicity assays. Collectively, these results indicate that rapid assessment of genotoxicity can be accomplished with a relatively simple flow cytometric technique, and that the scoring system is transferable across laboratories. Furthermore, a concurrent assessment of cytotoxicity, Flow-NBR, may help reduce the occurrence of irrelevant positive results, as it may represent a more appropriate means for choosing top concentration levels. Finally, the data presented herein reinforce concerns about the manner in which cytotoxicity limits are described in guidance documents, since these recommendations tend to cite fixed cut-off values without reference to methodology.
Keywords: Micronuclei, Automation, Flow cytometry, Genotoxicity, Cytotoxicity, L5178Y
1. Introduction
The in vitro micronucleus test is widely used as a screening assay to assess cytogenetic damage in mammalian cells [1–5]. Its popularity is largely due to the fact that it can be executed with less technical expertise relative to chromosome aberration analyses, and because it is capable of detecting aneugens as well as clastogens. Furthermore, there have been discussions that this assay system may serve a useful role in the international regulatory testing environment, and OECD guidelines are currently being developed [6]. Thus, there has been strong interest in automating the measurement of the in vitro micronucleus endpoint, a goal that could potentially reduce labor and costs, while enhancing throughput and scoring objectivity. In addition to the benefits of higher throughput capacity, some multi-parametric analysis platforms offer the possibility of integrating cytotoxicity endpoint(s) into the assay, thereby helping to avoid/reduce the risk of irrelevant positive results.
We have previously reported on the development of a scoring system that may enhance the utility of a flow cytometric procedure originally described by Nüsse and colleagues [7–11]. Most importantly, the new method includes an ethidium monoazide bromide (EMA) staining step in order to label the chromatin of necrotic and mid/late stage apoptotic cells. Subsequent stripping of cytoplasmic membranes and incubation with the pan-nucleic acid dye SYTOX Green (plus RNase) provides a suspension of free nuclei and micronuclei (MN) that are differentially stained relative to chromatin associated with dead/dying cells. Using this method, good agreement between microscopy- and flow cytometry-based scoring was reported for mouse L5178Y and human TK6 cells [12–13].
The experiments described herein where designed to further evaluate this MN scoring system by testing its transferability across laboratories. Another chief aim was to assess the merits of flow cytometric measurements of cytotoxicity, especially one that provides a relative survival-like measurement simultaneously with the MN scoring process. This method utilizes latex particles as counting beads, whereby flow cytometric nuclei to bead ratio measurements are used to calculate relative survival (hereafter this method is abbreviated Flow-NBR). Importantly, the measurement is a multi-parametric assessment of cell health, as it requires nuclei to be EMA-negative, and also to exhibit forward and side scatter characteristics of healthy nuclei. Based on our previous work with this endpoint [13], we hypothesize that the Flow-NBR will tend to be a more sensitive indicator of cytotoxicity than most widely utilized methods. Experiments described herein included several additional cytotoxicity assays that served as a basis for evaluating the reliability and sensitivity of the Flow-NBR endpoint.
For these experiments, L5178Y cells were treated with the diverse genotoxic agents mitomycin C (MMC), etoposide (ETOPO), and vinblastine (VB), or the presumed non-genotoxicants sucrose (SUC), staurosporine (STS), and dexamethasone (DEX), the latter two of which are known to exhibit potent apoptogenic activity. In the current trial, the treatment scheme was 24 hrs of continuous treatment. Resulting flow cytometric data are presented, along with parallel microscopy-based MN measurements. Indices of cytotoxicity are presented, as well as a discussion of the disparate results that occur when different methods are used to guide top concentration selection. Table I provides an overview of the participating laboratories, the equipment utilized, and the various cytotoxicity assays that were evaluated.
Table I.
Participating Laboratories.
| Site | Lab Code | Chemicals | Cytotoxicity Measurements | Flow Cytometer | Microscopy |
|---|---|---|---|---|---|
| Litron Laboratories, Rochester, NY | L1 | SUC, DEX, STS, MMC, VB, and ETOPO | RS: Flow-NBR RS: Fluorescein diacetate RS: Coulter Counter Population Doubling %EMA+ Events |
BD FacsCalibur running CellQuest v3.3 |
Olympus BH2, 10 × 40 Mag., Acridine orange staining |
| Novartis Pharma AG, Basel, Switzerland | L2 | SUC, MMC | RS: Flow-NBR RS: Trypan blue, Schilling camera RS: Sysmex Counter Population Doubling %EMA+ Events |
BD FacsCalibur running CellQuest Pro v4.0.2 |
Zeiss, 10 × 100x Mag., Feulgen + Congo Red staining |
| Johnson & Johnson, Pharmaceutical R&D, Beerse, Belgium | L3 | STS, ETOPO | RS: Flow-NBR RS: Coulter Counter RS: NucleoCounter Population Doubling %EMA+ Events |
BD FacsCalibur running CellQuest Pro v5.2 |
Zeiss Axioskop, 1.25 × 63 Mag., Giemsa staining |
| GlaxoSmithKline R&D, Hertfordshire, UK | L4 | DEX, VB | RS: Flow-NBR RS: Coulter Counter Population Doubling %EMA+ Events Annexin Viability |
BD FacsCalibur running CellQuest Pro v5.2.1 |
Leica DMR XA, 1.25 × 40 Mag., Acridine orange staining |
Abbreviations: SUC = sucrose; DEX = dexamethasone; STS = staurosporine; MMC = mitomycin C; VB = vinblastine sulfate; ETOPO = etoposide; RS = relative survival; EMA = ethidium monoazide bromide; Flow-NBR = flow cytometric nuclei to bead ratio
2. Materials and Methods
2.1. Reagents
Test chemicals were purchased from Sigma-Aldrich. Reagents needed to stain and lyse cells for flow cytometric analysis were supplied to all participating labs in kit format (Prototype In Vitro MicroFlow™ Kit, v0610.25, Litron Laboratories, Rochester, NY). These materials included Nuclei Acid Dye A Solution (contains EMA dye), Nuclei Acid Dye B Solution (contains SYTOX Green dye), Lysis Solutions 1 and 2, and RNase Solution. 6 μm fluorescent beads were used as counting beads, and were obtained from Invitrogen (cat. no. P14828).
2.2. Cells and culture medium
L5178Y cells were grown in culture medium at 37°C, 5% CO2, and in a humid atmosphere. For routine culturing, the cells were maintained between approximately 1 × 104 and 1 × 106 cells/ml. The culture medium consisted of RPMI 1640, to which heat-inactivated horse serum was added for 10% v/v final concentration. The sources of RPMI 1640 were Mediatech, Inc. (lab L1) and Invitrogen (labs L2, L3, and L4). The suppliers of horse serum were Invitrogen (L1 and L2) and BioWhittaker-Cambrex and Biosera (L3 and L4, respectively). All labs included L-glutamine in their culture medium, which was purchased from Mediatech by L1 or Invitrogen by L2, L3 and L4. Antibiotics were also included in culture medium: penicillin and streptomycin at L1 were from Mediatech; penicillin and streptomycin at L2 and L4 were from Invitrogen; gentamicine at L3 was from Invitrogen. Sodium pyruvate was added to RPMI by L1 (Sigma-Aldrich) and L4 (Invitrogen); L2 added HEPES (Invitrogen); and L3 added sodium bicarbonate and sodium pyruvate (both from Invitrogen).
2.3. Chemical Treatment
Prior to treatment, logarithmically growing cells were adjusted to 1.5 × 105/ml with culture medium, and 10 ml aliquots were transferred to T25 culture flasks. All treatments occurred in duplicate in the presence of 10% serum. Stock solutions were prepared such that a consistent volume of solvent was added to each flask (from 100x solutions of SUC or MMC, prepared in water; or from 1000x solutions of DEX, VB, ETOPO, or STS, prepared in dimethyl sulfoxide). In all cases, cells were incubated at 37°C for 24 hrs (the length of time that is approximately 2.5 doubling times for untreated cells, as recommended by an expert working group [1]).
2.4. Cytotoxicity Measurements
2.4.1. Coulter counter
At the 24 hr harvest time, cells were resuspended by pipetting each culture several times. Immediately after pipetting, 500 μl were transferred to cuvettes containing 9.5 ml of an isotonic salt solution. Cell densities were measured with Coulter counters, for instance model ZM at lab L1. Relative survival calculations were made based on the mean concurrent solvent control cell density. As suggested by an expert working group and also draft OECD guidances [1, 6], concentrations that resulted in > 60% reduction to relative survival were excluded from MN assessment.
L2 did not use a Coulter counter, but rather a Sysmex Microcellcounter (F-500, Switzerland). In this case, cells were resuspended by pipetting and 100 μl were transferred to cuvettes containing 5 ml of Cell Pack Sysmex buffer. As with Coulter counts, relative survival was calculated as a percentage of concurrent solvent control cultures.
In addition to supplying the data necessary to calculate relative survival, Coulter and Sysmex data were also used to calculate the number of population doublings (PDs) as described by Greenwood et al. [14]. These calculations were made as follows: PD = [log(Ni/N0)]/log 2, where N0 = baseline count at time zero; Ni = cell concentration at a given time point, i. To facilitate comparisons with other cytotoxicity endpoints, we expressed PDs as a percentage observed for the concurrent solvent control.
2.4.2. Relative survival based on nuclei to bead ratios (Flow-NBR)
To each culture deemed to exhibit an acceptable level of cytotoxicity based on Coulter/Sysmex counts (i.e., mean relative survival ≥ 40%), a 500 μl aliquot of counting bead solution was added. (This bead suspension was prepared by adding 4 – 6 drops of stock 6 μm beads to 10 ml culture medium.) As counting beads were present throughout the remaining cell processing steps, nuclei to bead ratios could be determined for each sample, and were used to calculate flow cytometry-based relative survival measurements. As noted previously, only nuclei that exhibited healthy cells’ light scatter and EMA-negative staining characteristics were included in these Flow-NBR measurements. As with the Coulter counts, these relative survival calculations were based on concurrent mean solvent control values.
2.4.3. Ethidium monoazide staining (EMA)
As described previously [12–13], in the course of performing flow cytometry-based MN measurements, the frequency of EMA-positive events is obtained. The EMA-positive phenotype is acquired upon loss of membrane integrity, and occurs during necrosis, and also mid- to late-stage apoptosis [12]. In the present investigation, the statistic is expressed as the percentage of EMA-positive chromatin as a fraction of all chromatin. Note that this calculation includes all chromatin with a SYTOX fluorescent signal of ≥ 1/100 of the G1 peak, and is therefore particularly sensitive to the presence of sub-2n particles that are generated during apoptosis.
2.4.4. Fluorescein diacetate staining (FDA)
For those cultures that exhibited mean relative survival values ≥ 40% based on Coulter counting, L1 transferred 500 μl of resuspended cells to flow cytometry tubes containing an equal volume of phosphate buffered saline solution containing 0.075 μg/ml fluorescein diacetate (FDA) and 25 μg/ml propidium iodide (PI). Since the cell culture included 6 μm counting beads at this point (see section 2.4.2.), the number of presumably live cells (FDA-positive, PI-negative) could be determined through flow cytometric analysis. As with the Coulter and Flow-NBR counts, these relative survival calculations were based on concurrent mean solvent control values.
2.4.5. Trypan blue
At the 24 hr harvest time, L2 transferred 200 μl of resuspended cells to 5 ml polystyrene tubes containing 200 μl Trypan Blue solution (0.4% Trypan blue solution, Sigma, Germany) that had been prediluted with an equal volume of phosphate buffered saline solution (Oxoid, England). The suspension was vortexed, placed in a Schilling Camera (Assistant, Germany) and the number of viable cells was estimated with a light microscope (Zeiss, Germany). Relative survival was calculated as a percentage of concurrent solvent control cultures.
2.4.6. NucleoCounter
At the 24 hr harvest time, lab L3 transferred an aliquot of resuspended cells to a NucleoCounter® (Chemometec, Denmark). This device provides automated cell counting of mammalian cell cultures, and is equipped with an integrated fluorescence microscope, a CCD camera and hardware-based image analysis. With this method, cells were lysed and sampled by a disposable device, the NucleoCassette™, which is internally pre-coated with the fluorescent nucleic acid dye propidium iodide. The total number of nuclei was quantified, and for the purposes of this presentation, NucleoCounter values are expressed as a percentage of the concurrent solvent controls.
2.4.7. Annexin staining
At the 24 hr harvest time, L4 determined cell viability as described in the BD Pharmingen™ Technical Datasheet for the Annexin V-FITC Apoptosis Detection Kit I (BD Biosciences, UK). Flow cytometry was used to measure the percentage of viable cells (i.e., Annexin-and propidium iodide-negative cells). These values are expressed as a percentage of viable cells relative to concurrent solvent controls.
2.5. Flow cytometric scoring of micronuclei
2.5.1. Cell Staining
Each laboratory accomplished flow cytometry-based MN measurements with reagents and instructions found in the Prototype In Vitro MicroFlow Kit (Litron Laboratories, Rochester, NY). Briefly, for each culture that exhibited ≥ 40% relative survival based on Coulter (or Sysmex) counter data, 5 × 105 total cells were transferred to 15 ml centrifuge tubes. Cells were collected via centrifugation at approximately 300 × g for 5 min. Supernatants were aspirated, cells were resuspended, and 300 μl of kit-supplied Nucleic Acid Dye A Solution were added. These tubes were placed in racks and submerged to a depth of approximately 2 cm in crushed ice. A fluorescent light bulb was positioned approximately 15 cm above the specimens for 30 min.
After the photoactivation period, 3 ml of cold kit-supplied Buffer Solution were added to each sample. The cells were collected via centrifugation, and the supernatants aspirated such that approximately 50 μl of supernatant remained per tube. The cells were gently resuspended, and then 500 μl of kit-supplied Lysis Solution 1 were added to each tube, which was immediately vortexed. These samples were kept at room temperature for 1 hr. At this time, 500 μl of kit-supplied Lysis Solution 2 were injected forcefully into each tube, which was then immediately vortexed. These samples were stored at 4°C until flow cytometric analysis occurred (within three days).
2.5.2. Instrumentation and gating
Strategies for acquiring flow cytometric data, including configuration of regions and gating logic, were as described in the Prototype In Vitro MicroFlow Kit manual, and as described by Bryce et al. [13]. Briefly, data acquisition was accomplished with flow cytometers providing 488 nm excitation. SYTOX-associated fluorescence emission was collected in the FL1 channel (530/30 band-pass filter), and EMA-associated fluorescence was collected in the FL3 channel (670 long-pass filter). Events were triggered on FL1 fluorescence. The incidence of MN was determined through the acquisition of at least 20,000 gated nuclei per culture.
2.6. Micronucleus scoring via microscopy
The microscopes and stains used to perform micronucleus counts at each site are described in Table I. For each culture that exhibited relative survival ≥ 40% (based on Coulter/Sysmex counts), 2000 mononucleated, non-apoptotic, non-necrotic cells were examined for the presence of MN (2000 cells per culture × duplicate cultures = 4000 cells per chemical concentration). In order to be scored as an MN-containing cell, the inclusion body had to be approximately round in shape, exhibit similar staining characteristics as the main nucleus, be less than 1/3 the size of the main nucleus, and could touch but not overlap the main nucleus (i.e., HUMN criteria, [15]).
2.7. Data analysis
All frequencies, averages, and fold-increase calculations were made with Excel Office X for Mac® (Microsoft, Seattle, WA). We utilized a simple a priori approach to signify negative and positive responses. That is, significant induction of MN events was taken to be ≥ 3-fold mean increase over concurrent negative control. An advantage of a fold increase judgment is that it served a normalization role, facilitating comparisons between labs and also across scoring methods. While more formal statistical methods are often applied to in vitro MN data, they usually also consider historical negative control data so that statistical significance is not the only criterion for a positive call. The establishment of substantial historical control data at each site was deemed beyond the scope of this initial assessment of assay portability, leading to our fold-increase approach for this study.
Previous data have suggested that Flow-NBR may reveal cytotoxicity that other approaches do not [13]. Therefore, for each experiment described herein, data are interpreted in the context of two strategies for eliminating overly toxic concentrations from consideration. More specifically, excessive toxicity was indicated by mean relative survival values < 40% based on Coulter/Sysmex counts, or alternately, on the Flow-NBR measurements. Data from several other endpoints of cytotoxicity are presented for comparison purposes and additional consideration.
When either flow cytometry- or microscopy-based data suggested that significant MN induction had occurred (i.e., ≥ 3-fold increase, top concentration based on Flow-NBR results), Spearman correlation coefficients (rs) were calculated to assess agreement between methods and across laboratories (JMP software, v5.0, SAS Institute, Inc., Cary, NC). Specifically, this statistic was calculated for flow cytometry-based %MN values between labs, microscopy-based %MN values between labs, and flow cytometry- versus microscopy-based %MN values within and between labs.
3. Results
3.1. Initial considerations
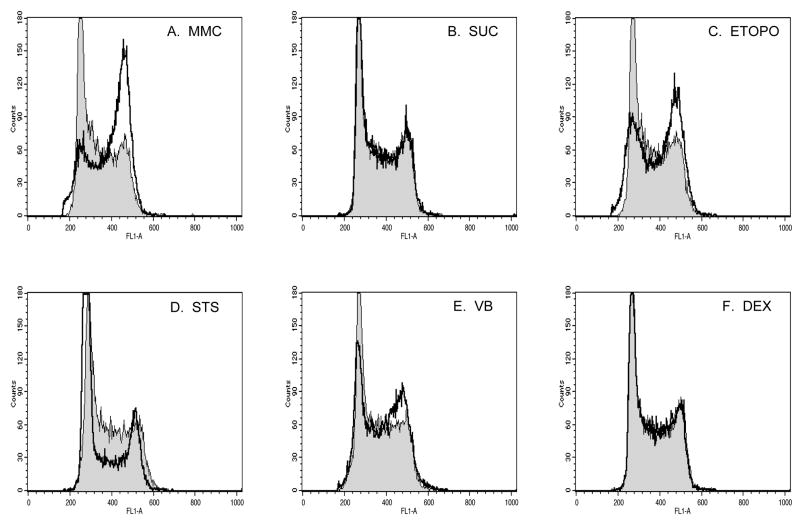
The procedures for flow cytometric scoring of MN were found to be transferable across labs, as each participating site observed a high degree of resolution among MN events, nuclei, and the chromatin of dead/dying cells (an example of which is shown in Fig 1). Two observations made during initial trials are worth noting. First, one group (L3) recorded unusually low baseline MN frequencies. These investigators were advised to obtain a new batch of cells from ATCC, which provided higher MN frequencies that fell into the range observed at the other sites. A decision was made to use the newer cells for the definitive experiments described below. Second, although all groups utilized the same model flow cytometer, it was necessary for L4 to adjust the angle of their forward light scatter versus FL1 region (Fig 1D), in order to better encompass MN events.
Figure 1.
Histogram and bivariate plots of solvent treated L5178Y cells. Panels A–F illustrate the gating strategy used to discriminate micronuclei from apoptotic and necrotic chromatin, as well as other subcellular debris. In order for events to be displayed on the nuclei and micronuclei scoring plot (Panel G), they needed to meet each of the following criteria: within a side scatter vs. forward scatter region, panel A; within a region that excludes doublets, plot B; at least 1/100 the SYTOX-associated fluorescence of G1 nuclei, panel C; within a forward scatter vs. SYTOX fluorescence region, plot D; within a side scatter vs. SYTOX fluorescence region, plot E; and EMA-negative, panel F. Panel G illustrates the micronucleus scoring region (MN), which was configured to score events that exhibited 1/100 to 1/10 the SYTOX fluorescence intensity of G1 nuclei. Panel H plots nuclei that met light scatter and EMA-negative characteristics as described above. These data were used to qualitatively assess cell cycle perturbations (Fig 3).
3.2. Mitomycin C
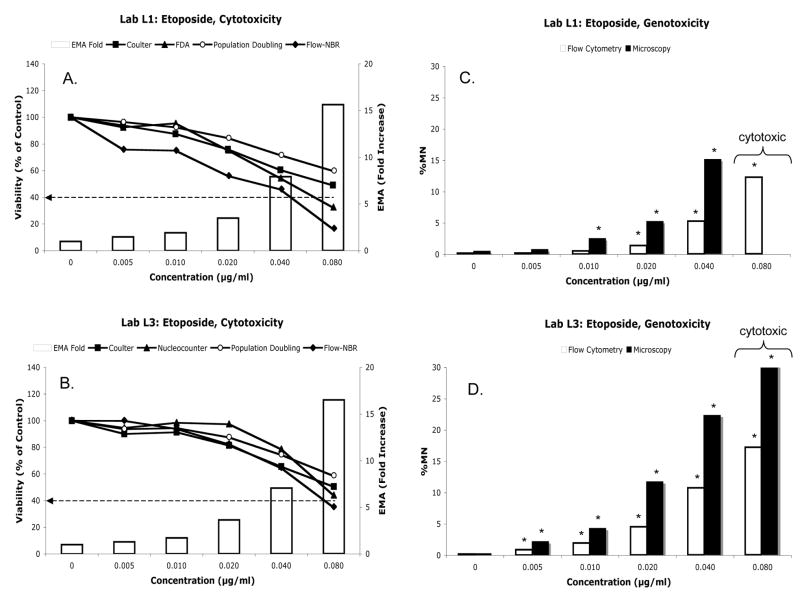
Cytotoxicity measurements associated with MMC treatment are shown in Fig 2A–B. Based on Coulter or Sysmex counts, L1 and L2 found that the highest concentration of MMC tested (0.3125 μg/ml) was not overly cytotoxic, as mean relative survival values were greater than 50%. However, relative survival measurements of these same cultures were lower than the 40% cut off value when based on the Flow-NBR approach. Two cytotoxicity assessments that also provided relative survival measures less than 40%, trypan blue- and FDA-staining, also share the characteristic of being able to exclude dead/dying cells from consideration.
Figure 2.
Mitomycin C data: cytotoxicity (panels A and B) and genotoxicity (panels C and D). Panels A and B: Lines correspond to each of several cytotoxicity endpoints whose data are expressed as percent viability relative to solvent control. These data should be read on the Y-axis. White bars correspond to EMA-fold increase data, and these cytotoxicity measurements should be read on the YY-axis. For convenience, a dashed line has been added to denote the 40% relative survival value that has been cited as an appropriate means for choosing top concentration. Panels C and D: Fold increase in mean micronuclei frequencies as measured by flow cytometry (white bars) and by microscopy (black bars) are shown. Asterisks (*) signify ≥ 3-fold micronucleus induction. Brackets indicate concentrations that were deemed overly cytotoxic by the Flow-NBR technique.
The effect of MMC on the cell cycle was qualitatively consistent between laboratories. That is, MMC was observed to cause a pronounced accumulation of nuclei in G2/M (see Fig 3A), even at the lowest concentration studied (0.039 μg/ml).
Figure 3.
In addition to micronucleus counts, flow cytometric analysis simultaneously provides cell cycle information based on the SYTOX-associated fluorescence of detergent-liberated nuclei. The grey-filled histograms correspond to a solvent control, while the superimposed black lines correspond to each of the six test articles (at the highest concentration that was deemed not overly cytotoxic by the Flow-NBR method). Note that these are gated nuclei, meaning they had to exhibit light scatter characteristics and the EMA-negative phenotype of healthy nuclei, as described in Fig 1.
As shown in Fig 2C–D and Table IIA, both laboratories found dose-related increases in MN when measured by flow cytometry or microscopy. While %MN values tended to be highest when measurements were made at L2 via flow cytometry, there was generally good agreement between methods and between laboratories, with rs values ranging from 0.9524 to 0.9762 (Table IIIA).
Table II.
| Table II. A. Micronucleus Data: Mitomycin C. | ||||||||
|---|---|---|---|---|---|---|---|---|
| %MN: Microscopy | %MN: Flow |
|||||||
| Chemical | Lab | Conc. | Values | Mean | Fold Increase | Values | Mean | Fold Increase |
| Mitomycin C | L1 | 0 | 0.4, 0.3 | 0.35 | - | 0.15, 0.16 | 0.16 | - |
| 0.039 | 3.5, 2.9 | 3.20 | 9.1 | 1.86, 1.46 | 1.66 | 10.4 | ||
| 0.078 | 6.1, 5.3 | 5.70 | 16.3 | 4.54, 3.72 | 4.13 | 25.8 | ||
| 0.156 | 13.2, 8.5 | 10.85 | 31.0 | 7.47, 6.76 | 6.87 | 42.9 | ||
| 0.3125 | 19.7, 20.7 | 20.20 | 57.7 | 11.75, 17.05 | 14.40 | 90.0 | ||
| L2 | 0 | 0.5, 0.2 | 0.35 | - | 0.12, 0.09 | 0.11 | - | |
| 0.039 | 4.6, 3.8 | 4.20 | 12.0 | 4.01, 4.00 | 4.00 | 36.4 | ||
| 0.078 | 4.7, 6.0 | 5.35 | 15.3 | 10.79, 8.40 | 9.60 | 87.3 | ||
| 0.156 | 8.0, 7.6 | 7.80 | 22.3 | 15.16, 15.48 | 15.32 | 139.3 | ||
| 0.3125 | 8.2, 8.5 | 8.35 | 23.9 | 15.99, 17.43 | 16.71 | 151.9 | ||
| Table II. B. Micronucleus Data: Sucrose. | ||||||||
|---|---|---|---|---|---|---|---|---|
| %MN: Microscopy | %MN: Flow |
|||||||
| Chemical | Lab | Conc. | Values | Mean | Fold Increase | Values | Mean | Fold Increase |
| Sucrose | L1 | 0 | 0.2, 0.1 | 0.15 | - | 0.18, 0.20 | 0.19 | - |
| 625 | 0.3, 0.3 | 0.30 | 2.0 | 0.14, 0.22 | 0.18 | 0.9 | ||
| 1250 | 0.1, 0.2 | 0.15 | 1.0 | 0.20, 0.11 | 0.16 | 0.8 | ||
| 2500 | 0.5, 0.3 | 0.40 | 2.7 | 0.19, 0.17 | 0.18 | 0.9 | ||
| 5000 | 0.4, 0.1 | 0.25 | 1.7 | 0.20, 0.30 | 0.25 | 1.3 | ||
| L2 | 0 | 0.2, 0.1 | 0.15 | - | 0.22, 0.27 | 0.25 | - | |
| 625 | 0.2, 0.2 | 0.20 | 1.3 | 0.31, 0.13 | 0.22 | 0.9 | ||
| 1250 | 0.3, 0.3 | 0.30 | 2.0 | 0.34, 0.11 | 0.23 | 0.9 | ||
| 2500 | 0.1, 0.3 | 0.20 | 1.3 | 0.51, 0.36 | 0.44 | 1.8 | ||
| 5000 | 0.2, 0.3 | 0.25 | 1.7 | 0.31, 0.18 | 0.25 | 1.0 | ||
| Table II. C. Micronucleus Data: Etoposide. | ||||||||
|---|---|---|---|---|---|---|---|---|
| %MN: Microscopy | %MN: Flow |
|||||||
| Chemical | Lab | Conc. | Values | Mean | Fold Increase | Values | Mean | Fold Increase |
| Etoposide | L1 | 0 | 0.4, 0.4 | 0.40 | - | 0.15, 0.21 | 0.18 | - |
| 0.005 | 0.6, 0.7 | 0.65 | 1.6 | 0.23, 0.10 | 0.17 | 0.9 | ||
| 0.01 | 2.4, 2.4 | 2.40 | 6.0 | 0.50, 0.49 | 0.50 | 2.8 | ||
| 0.02 | 5.2, 5.1 | 5.15 | 12.9 | 1.29, 1.43 | 1.36 | 7.6 | ||
| 0.04 | 15.8, 14.3 | 15.05 | 37.6 | 5.19, 5.28 | 5.24 | 29.1 | ||
| 0.08 | ND | ND | ND | 13.07, 11.49 | 12.28 | 68.2 | ||
| L3 | 0 | 0.2, 0.1 | 0.15 | - | 0.16, 0.14 | 0.15 | - | |
| 0.005 | 2.1, 2.1 | 2.10 | 14.0 | 1.08, 0.66 | 0.87 | 5.8 | ||
| 0.01 | 3.9, 4.5 | 4.20 | 28.0 | 2.04, 1.81 | 1.93 | 12.9 | ||
| 0.02 | 10.7, 12.6 | 11.65 | 77.7 | 4.76, 4.22 | 4.49 | 29.9 | ||
| 0.04 | 22.2, 22.4 | 22.30 | 148.7 | 11.16, 10.28 | 10.72 | 71.5 | ||
| 0.08 | 32.4, 31.1 | 31.75 | 211.7 | 16.81, 17.65 | 17.23 | 114.9 | ||
| Table II. D. Micronucleus Data: Staurosporine. | ||||||||
|---|---|---|---|---|---|---|---|---|
| %MN: Microscopy | %MN: Flow |
|||||||
| Chemical | Lab | Conc. | Values | Mean | Fold Increase | Values | Mean | Fold Increase |
| Staurosporine | L1 | 0 | 0.2, 0.1 | 0.15 | - | 0.18, 0.24 | 0.21 | - |
| 0.0016 | 0.1, 0.3 | 0.20 | 1.3 | 0.12, 0.12 | 0.12 | 0.6 | ||
| 0.0031 | 0.5, 0.4 | 0.35 | 2.3 | 0.07, 0.16 | 0.12 | 0.6 | ||
| 0.0062 | 0.3, 0.5 | 0.40 | 2.7 | 0.10, 0.17 | 0.14 | 0.7 | ||
| 0.0125 | 0.3, 0.3 | 0.30 | 2.0 | 0.30, 0.28 | 0.29 | 1.4 | ||
| 0.0250 | ND | ND | ND | 0.93, 1.53 | 1.23 | 5.9 | ||
| L3 | 0 | 0.2, 0.3 | 0.25 | - | 0.17, 0.14 | 0.16 | - | |
| 0.0016 | 0.2, 0.3 | 0.25 | 1.0 | 0.14, 0.08 | 0.11 | 0.7 | ||
| 0.0031 | 0.6, 0.4 | 0.50 | 2.0 | 0.20, 0.17 | 0.19 | 1.2 | ||
| 0.0062 | 1.1, 1.0 | 1.05 | 4.2 | 0.23, 0.23 | 0.23 | 1.4 | ||
| 0.0125 | 1.1, 0.8 | 0.95 | 3.8 | 0.27, 0.37 | 0.32 | 2.0 | ||
| 0.0250 | 1.1, 0.7 | 0.90 | 3.6 | 0.74, 1.07 | 0.91 | 5.7 | ||
| Table II. E. Micronucleus Data: Vinblastine. | ||||||||
|---|---|---|---|---|---|---|---|---|
| %MN: Microscopy | %MN: Flow |
|||||||
| Chemical | Lab | Conc. | Values | Mean | Fold Increase | Values | Mean | Fold Increase |
| Vinblastine | L1 | 0 | 0.1, 0.1 | 0.10 | - | 0.26, 0.21 | 0.24 | - |
| 0.9 | 0.1, 0.3 | 0.20 | 2.0 | 0.36, 0.76 | 0.56 | 2.3 | ||
| 1.0 | 0.5, 0.4 | 0.45 | 4.5 | 0.58, 0.71 | 0.65 | 2.7 | ||
| 1.1 | 0.7, 1.0 | 0.85 | 8.5 | 1.03, 1.98 | 1.51 | 6.3 | ||
| 1.2 | 0.8, 1.3 | 1.05 | 10.5 | 1.16, 1.82 | 1.49 | 6.2 | ||
| 1.3 | 1.2, 1.4 | 1.30 | 13.0 | 6.31, 2.36 | 4.34 | 18.1 | ||
| L4 | 0 | 0.2, 0.3 | 0.25 | - | 0.16, 0.10 | 0.13 | - | |
| 0.9 | 0.4, 0.3 | 0.35 | 1.4 | 0.19, 0.23 | 0.21 | 1.6 | ||
| 1.0 | 0.6, 0.5 | 0.55 | 2.2 | 0.35, 0.33 | 0.34 | 2.6 | ||
| 1.1 | 0.6, 0.6 | 0.60 | 2.4 | 0.51, 0.51 | 0.51 | 3.9 | ||
| 1.2 | 0.7, 1.2 | 0.95 | 3.8 | 0.90, 1.34 | 1.12 | 8.6 | ||
| 1.3 | 0.7, 1.7 | 1.20 | 4.8 | 1.96, 1.54 | 1.75 | 13.5 | ||
| Table II. F. Micronucleus Data: Dexamethasone. | ||||||||
|---|---|---|---|---|---|---|---|---|
| %MN: Microscopy | %MN: Flow |
|||||||
| Chemical | Lab | Conc. | Values | Mean | Fold Increase | Values | Mean | Fold Increase |
| Dexamethasone | L1 | 0 | 0.2, 0.1 | 0.15 | - | 0.28, 0.29 | 0.28 | - |
| 7.8 | 0.3, 0.4 | 0.35 | 2.3 | 0.20, 0.36 | 0.28 | 1.0 | ||
| 15.625 | 0.2, 0.3 | 0.25 | 1.7 | 0.29, 0.35 | 0.32 | 1.1 | ||
| 31.25 | 0.3, 0.5 | 0.40 | 2.7 | 0.58, 0.43 | 0.50 | 1.8 | ||
| 62.5 | 0.3, 0.6 | 0.50 | 3.3 | 3.32, 1.84 | 2.08 | 7.3 | ||
| L4 | 0 | 0.2, 0.4 | 0.30 | - | 0.62, 0.34 | 0.48 | - | |
| 15.625 | 0.4, 0.5 | 0.45 | 1.5 | 0.17, 0.65 | 0.41 | 0.9 | ||
| 31.25 | 0.3, 0.5 | 0.40 | 1.3 | 0.31, 0.23 | 0.27 | 0.6 | ||
| 62.5 | 0.3, 0.2 | 0.25 | 0.8 | 0.56, 0.45 | 0.50 | 1.0 | ||
| 125 | 0.4, 0.6 | 0.50 | 1.7 | 1.86, 2.15 | 2.01 | 4.2 | ||
Note: shading indicates concentration is excessively cytotoxic based on Flow-NBR assessment.
ND = not determined; too cytotoxic.
Table III.
| Table III. A. Spearman Correlation Coefficients: Mitomycin C. | |||
|---|---|---|---|
| Lab L1, Microscopy | Lab L1, Flow Cytometry | Lab L2, Flow Cytometry | |
| Lab L1, Microscopy | ——— | ——— | 0.9762 |
| Lab L1, Flow Cytometry | 0.9762 | ——— | 0.9524 |
| Lab L2, Microscopy | 0.9762 | 0.9524 | 0.9524 |
| Table III. B. Spearman Correlation Coefficients: Etoposide. | |||
|---|---|---|---|
| Lab L1, Microscopy | Lab L1, Flow Cytometry | Lab L3, Flow Cytometry | |
| Lab L1, Microscopy | ——— | ——— | 0.9817 |
| Lab L1, Flow Cytometry | 0.8964 | 0.9273 | |
| Lab L3, Microscopy | 0.9664 | 0.9240 | 0.9605 |
| Table III. C. Spearman Correlation Coefficients: Staurosporine. | |||
|---|---|---|---|
| Lab L1, Microscopy | Lab L1, Flow Cytometry | Lab L3, Flow Cytometry | |
| Lab L1, Microscopy | ——— | ——— | 0.4074 |
| Lab L1, Flow Cytometry | −0.2742 | ——— | 0.3650 |
| Lab L3, Microscopy | 0.5485 | 0.1166 | 0.8086 |
| Table III. D. Spearman Correlation Coefficients: Vinblastine. | |||
|---|---|---|---|
| Lab L1, Microscopy | Lab L1, Flow Cytometry | Lab L4, Flow Cytometry | |
| Lab L1, Microscopy | ——— | ——— | 0.9662 |
| Lab L1, Flow Cytometry | 0.9265 | 0.8997 | |
| Lab L4, Microscopy | 0.9159 | 0.7755 | 0.9507 |
3.3. Sucrose
Cytotoxicity measurements for SUC are presented in Fig 4A–B. Each of the several methods utilized showed that the highest concentration tested (5,000 μg/ml) was not cytotoxic to L5178Y cells. Furthermore, SYTOX fluorescence profiles demonstrated that SUC had no effect on the cell cycle (Fig 3B). No evidence of MN induction was detected, irrespective of scoring method (Fig 4C–D and Table IIB).
Figure 4.
Sucrose data: cytotoxicity (panels A and B) and genotoxicity (panels C and D). Format is same as Figure 2.
3.4. Etoposide
Cytotoxicity measurements associated with ETOPO treatment are provided in Fig 5A–B. When basing the judgment on Coulter counts, the highest concentration of ETOPO tested (0.08 μg/ml) did not appear to be overly cytotoxic, as mean relative survival values were above 40%. Conversely, excessive cytotoxicity was indicated by Flow-NBR measurements at both labs. Furthermore, FDA and %EMA data suggested extreme cytotoxicity. For instance, L1 observed %EMA-positive events to increase by 8.0 and 15.8-fold at the 0.04 and 0.08 μg/ml concentrations, respectively. This may be related to the fact that the EMA-based statistic is sensitive to the presence of apoptotic bodies, a mode of cell death that was particularly evident for the two highest concentrations of ETOPO concentrations (as noted by L1 and L3 microscopists during slide-based MN scoring, data not shown).
Figure 5.
Etoposide data: cytotoxicity (panels A and B) and genotoxicity (panels C and D). Format is same as Figure 2.
The effect of ETOPO on the cell cycle was qualitatively consistent between laboratories. That is, ETOPO was observed to cause a dose-related accumulation of nuclei in the G2/M peak (Fig 3C).
MN data are presented in Fig 5C–D and Table IIC. Both laboratories found dose-related increases in MN when measured by flow cytometry or microscopy. While %MN values tended to be highest when measurements were made at L3 via flow cytometry, there was generally good agreement between methods and between laboratories, with rs values ranging from 0.8964 to 0.9817 (Table IIIB).
3.5. Staurosporine
Cytotoxicity measurements associated with STS treatment are provided in Fig 6A–B. Whereas L1’s Coulter data indicated that the top concentration (0.025 μg/ml) approached but did not exceed the cytotoxicity threshold value, L3’s Coulter-based assessment found this concentration to be overly cytotoxic. Both sites observed Flow-NBR cytotoxicity measurements to be more sensitive than Coulter counts. A number of other cellular toxicity endpoints also highlighted pronounced STS effects, especially for the 0.025 μg/ml concentration at lab L1, and for the 0.0125 and 0.025 μg/ml concentrations at L3. For instance, L3 observed the frequency of EMA-positive events to increase by 6.2- and 20.6-fold at the two highest concentrations tested.
Figure 6.
Staurosporine data: cytotoxicity (panels A and B) and genotoxicity (panels C and D). Format is same as Figure 2.
The effect of STS on the cell cycle was qualitatively consistent between laboratories. That is, STS was observed to cause a loss of cycling cells, particularly those with S-phase DNA content (Fig 3D).
MN data are shown in Fig 6C–D and Table IID. Both L1 and L3 observed elevated MN frequencies for the 0.025 μg/ml concentration when scoring was accomplished by flow cytometry. Alternately, when the concentration range was limited to ≤ 0.0125 μg/ml, as suggested by Flow-NBR data (as well as NucleoCounter and FDA staining), no significant MN induction was observed for flow cytometric analyses. L1’s microscopy-based analyses showed slightly elevated MN values at 0.0031 and 0.0062 μg/ml concentrations, although they were less than 3-fold. L3’s microscopy-based analyses found 3.9-, 3.4-, and 3.3-fold increases at the highest three concentrations tested, respectively. It is noteworthy that the L3 microscopist observed markedly elevated numbers of apoptotic cells on these slides (9.8-, 56-, and 127-fold higher incidence for the three highest concentrations relative to solvent control).
Spearman correlation coefficients were calculated, and are provided in Table IIIC. The generally low values reflect the fact that MN induction was observed at only one site and one method (i.e., L3/microscopy). Secondly, this calculation is expected to produce a low correlation coefficient when data are narrowly distributed in a baseline-like range.
3.6. Vinblastine
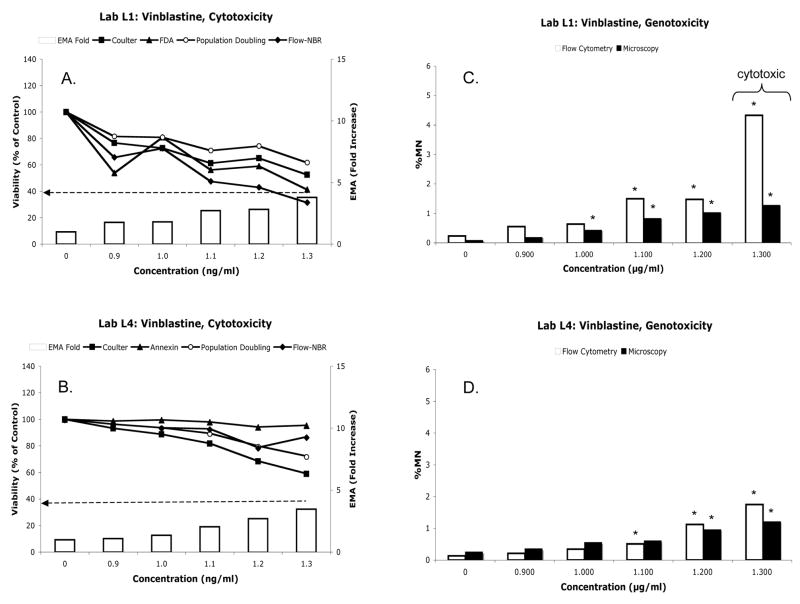
Cytotoxicity measurements associated with VB treatment are shown in Fig 7A–B. Based on Coulter counts, L1 and L4 determined that the highest concentration of VB tested (1.3 μg/ml) exhibited an acceptable level of cytotoxicity. On the other hand, L1’s Flow-NBR data suggested that the top concentration was overly toxic. FDA-based cytotoxicity data most closely paralleled the Flow-NBR profile, although the 40% relative survival threshold was approached, but not exceeded.
Figure 7.
Vinblastine data: cytotoxicity (panels A and B) and genotoxicity (panels C and D). Format is same as Figure 2.
VB was observed to perturb the cell cycle, as a modest accumulation of G2/M nuclei was apparent (Fig 3E). This finding was consistent between laboratories.
Genotoxicity data are shown in Fig 7C–D and Table IIE. Both laboratories observed significant induction of MN when measured by microscopy or flow cytometry. Agreement between methods and between laboratories was generally high, with rs values ranging from 0.7755 to 0.9662 (Table IIID).
3.7. Dexamethasone
DEX cytotoxicity data are provided in Fig 8A–B. L1’s Coulter data revealed unacceptable levels of cytotoxicity at the 62.5 μg/ml concentration. L4’s analogous assessment indicated that this cutoff value was approached but not exceeded by the 125 μg/ml concentration. At both sites, Flow-NBR data were found to be more sensitive to the cytotoxic effects of DEX, and suggested that concentrations should be restricted to 31.25 μg/ml in the case of L1, and 62.5 μg/ml in the case of L4. Data from FDA staining (L1), as well as the markedly elevated %EMA-positive events noted by both labs, provided further support that genotoxicity assessment should be limited to these concentrations.
Figure 8.
Dexamethasone data: cytotoxicity (panels A and B) and genotoxicity (panels C and D). Format is same as Figure 2.
DEX was not observed to have marked effects on cell cycle distribution when top concentration was guided by Flow-NBR cytotoxicity data (Fig 3F). On the other hand, perturbations were evident at overly cytotoxic concentrations. For instance, L4 observed marked accumulation of G2/M cells at 125 μg/ml.
MN data are presented in Fig 8C–D and Table IIF. Elevated MN frequencies were observed at the L1 site for the 62.5 μg/ml concentration, and at the L4 site for the 125 μg/ml concentration, when scoring was accomplished by flow cytometry. However, when the concentration range was based on Flow-NBR data, no significant MN induction was observed. Similarly, microscopy-based analyses at L1 and L4 showed no evidence of a genotoxic response, except for the 3.3-fold increase in MN observed at L1 for the overly cytotoxic concentration of 62.5 μg/ml.
4. Discussion
The poor assay specificity of mammalian cell genotoxicity tests has become increasingly well documented [18–19]. One of the chief reasons cited for the high rate of false positive results is the confounding effects of overtly cytotoxic concentrations [18–21]. Furthermore, when unoptimized automated scoring methods are utilized, further erosion of assay specificity can result. For instance, previous flow cytometry-based micronucleus scoring methods could not accurately measure MN frequency when significant levels of apoptosis occurred [10–11].
Yet the work undertaken here is based on the premise that the multi-parametric nature of flow cytometry technology represents a means for increasing specificity as well as addressing other issues. A case in point regards cytotoxicity assessment. As the current data suggest, carefully designed flow cytometry-based endpoints can reveal cytotoxicity that other methods tend to under represent. Thus, as demonstrated herein, flow cytometry helped limit top concentration more effectively than commonly used approaches such as cell counting. In addition, the use of EMA to label dead/dying cells’ chromatin demonstrates that carefully considered staining approaches can guard MN measurements against the confounding effects of apoptotic bodies or other spurious events. Thus, with this system in place, three genotoxicants and three presumed non-genotoxicants were characterized correctly. Collectively, these results support the concept that guidelines regarding cytotoxicity limits, as well as the methods used to accomplish these measurements, should be reconsidered [19, 22]. It seems likely that the implementation of such improvements could increase the meaningfulness of mammalian cell-based genotoxicity data.
It should be stressed that beyond providing an effective means for choosing top concentration and ensuring reliable MN enumeration, the multi-parametric flow cytometry platform supplied information regarding several forms of cellular damage. Thus, while the Flow-NBR statistic provided information on the number of viable cells, the incidence of EMA-positive events reflected loss of membrane integrity, light scatter characteristics conveyed information regarding chromatin condensation, and SYTOX-intensity reported cell cycle status. Thus, this “MN scoring technique” actually provides a comprehensive assessment of test article-induced effects. Such information aids data interpretation, and may represent a means for elucidating chemicals’ mode of action, a key component of safety assessment. Furthermore, reliance on a matrix of cytotoxicity endpoints such as this may prove beneficial because it helps address the concern that different methods can portray the extent of cytotoxicity quite differently [14, 22].
Six diverse chemical agents is not an exhaustive list of compounds. Clearly the number and classes of compounds analyzed needs to be expanded. Even so, these results are very encouraging, as the non-genotoxic cytotoxicants DEX and STS tend to be extreme challenges to mammalian cell genotoxicity assays [16–17]. Furthermore, we chose 24 hrs of continuous treatment as a particularly challenging experimental design to study, as previous work by lab L4 had demonstrated that assay specificity is reduced in this case [23]. Beyond expanding the number of chemical and treatment regimens evaluated, it will be important to continue these investigations along several lines:
Assay portability
Continue inter-laboratory studies in order to better characterize the transferability of the scoring procedure.
Compatibility with other cell lines
In addition to L5178Y cells, CHO [24–26], V79 [27], HepG2 [28], TK6 [29], and other cell lines have various advantageous characteristics. For instance, preliminary work with the attachment cell lines CHO-K1 and HepG2 indicate that the EMA dye and subsequent lysis solutions described herein can be directly applied to cells that are affixed to growth vessels, thereby liberating nuclei and MN without the need for trypsinization. The advantage of this is that cell processing occurs very efficiently, as all centrifugation steps can be eliminated. On the other hand, TK6 cells have the advantage of being of human origin, have a proficient p53 status and offer the possibility for combinational assays such as GreenScreen HC [30] or gene mutation analysis at the TK locus.
Scoring Characteristics
There is an important distinction between MN scoring that is accomplished by flow cytometry versus microscopy. Whereas microscopists base their MN measurements on the frequency of intact cells that contain these inclusion bodies, the flow cytometric approach described herein considers the frequency of MN-like particles relative to the number of free nuclei. Thus, some characteristics of flow-based measurements would be expected to yield higher MN frequencies. For instance when a cell contains two or more micronuclei, the flow cytometer registers these as separate events, whereas the microscopist scores this a single micronucleated cell. On the other hand, there are scoring characteristics that would be expected to increase MN frequencies for microscopy-based analyses relative to flow cytometry. For instance, binucleated cells and mitotic cells each contribute events that appear in the flow cytometric measurement’s denominator, whereas these events are not considered by microscopy (at least when cytochalasin B is not used, as was the case here). Thus, further experimentation will be required to understand whether certain culture conditions or chemical classes might tend to generate dissimilar MN values, or more importantly, dissimilar concentration response relationships, when these different scoring methods are utilized.
Apoptosis as a confounder
Reports by Mentieres et al. [16–17] and others suggest that the apoptotic program, perhaps especially nuclear fragmentation, can lead to the presence of MN-like events that may enhance the rate of false positive results. STS data presented here may represent a case in point, as elevated MN frequencies were observed by microscopy (L3). Conversely, no such induction was found with the flow cytometric procedure when concentrations were limited by Flow-NBR data, and when EMA staining was used to eliminate apoptotic chromatin from MN counts. Further work may be needed to develop a deeper understanding of the extent to which apoptosis and other manifestations of cytotoxicity can impact microscopy- and flow cytometry-based MN scoring techniques.
Miniaturization and further automation
The utility of this and other automated in vitro MN scoring systems will be magnified if and when they become miniaturized. Experiments described herein were performed with relatively large numbers and volumes of cells, which facilitated parallel microscopy and several cytotoxicity measurements per culture. However, significant reductions to numbers of cells and treatment volumes will be important for wide adoption of this or other techniques, especially in the context of lead prioritization and other early safety evaluation screening goals.
Screening programs that wish to evaluate large numbers of chemicals would also benefit from automation of other processing steps. For instance, flow cytometers that can autoload specimens from multi-well plates are now available. It will be important to assess whether specimens that have been generated according to the methodology described herein are compatible with such systems.
In summary, the reliability of rapid, flow cytometry-based scoring of in vitro micronuclei has been strongly supported, as these data have been consistent with microscopy-based measurements. The automated scoring methodology described herein offers certain advantages over manual and other MN scoring platforms, because in addition to MN frequency, it simultaneously provides several indices of cytotoxicity. As the data presented herein suggest, the high information content that is acquired is likely to prove valuable for selecting top concentration, as well as for elucidating mode of action.
Acknowledgments
This work was funded by a grant from the National Institute of Health/National Institute of Cancer (NCI) (S.D.D., No. 1R43CA117093-01A1). The contents are solely the responsibility of the authors, and do not necessarily represent the official views of the NCI.
Footnotes
Disclosure statement: Litron Laboratories has filed a patent covering flow cytometric methods for scoring micronuclei as described herein.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kirsch-Volders M, Sofuni T, Aardema M, Albertini S, Eastmond D, Fenech M, Ishidate M, Jr, Kirchner S, Lorge E, Morita T, Norppa H, Surrallés J, Vanhauwaert A, Wakata A. Report from the in vitro micronucleus assay working group. Mutat Res. 2003;540:153–163. doi: 10.1016/j.mrgentox.2003.07.005. [DOI] [PubMed] [Google Scholar]
- 2.Matsushima T, Hayashi M, Matsuoka A, Ishidate M, Jr, Miura KF, Shimizu H, Morimoto K, Ogura H, Mure K, Koshi K, Sufuni T. Validation study of the in vitro micronucleus test in a Chinese hamster lung cell line (CHL/IU) Mutagenesis. 1999;14:569–580. doi: 10.1093/mutage/14.6.569. [DOI] [PubMed] [Google Scholar]
- 3.Miller B, Potter-Locher F, Seelbach A, Stopper H, Utesch D, Madle S. Evaluation of the in vitro micronucleus test as an alternative to the in vitro chromosome aberration assay: position of the GUM working group on the in vitro micronucleus test. Mutat Res. 1998;410:81–116. doi: 10.1016/s1383-5742(97)00030-6. [DOI] [PubMed] [Google Scholar]
- 4.Lorge E, Thybaud V, Aardema MJ, Oliver J, Wakata A, Lorenzon G, Marzin D. SFTG international collaborative study on in vitro micronucleus test. I. General conditions and overall conclusions of the study. Mutat Res. 2006;607:13–36. doi: 10.1016/j.mrgentox.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 5.Lorge E, Lambert C, Gervais V, Becourt-Lhote N, Delongeas JL, Claude N. Genetic Toxicity Assessment: Employing the best science for human safety evaluation part II: Performances of the in vitro micronucleus test compared to the mouse lymphoma assay and the in vitro chromosome aberration assay. Tox Sci. 2007;96:214–217. doi: 10.1093/toxsci/kfl193. [DOI] [PubMed] [Google Scholar]
- 6.Organisation for Economic Co-operation and Development (OECD) Guidelines for the testing of chemicals, Draft Guideline 487: In vitro micronucleus test, December 21, 2006 (2nd version).
- 7.Nüsse M, Kramer J. Flow cytometric analysis of micronuclei found in cells after irradiation. Cytometry. 1984;5:20–25. doi: 10.1002/cyto.990050105. [DOI] [PubMed] [Google Scholar]
- 8.Schreiber GA, Beisker W, Bauchinger M, Nüsse M. Multiparametric flow cytometric analysis of radiation-induced micronuclei in mammalian cell cultures. Cytometry. 1992;13:90–102. doi: 10.1002/cyto.990130114. [DOI] [PubMed] [Google Scholar]
- 9.Wessels J, Nüsse M. Flow cytometric detection of micronuclei by combined staining of DNA and membranes. Cytometry. 1995;19:201–208. doi: 10.1002/cyto.990190303. [DOI] [PubMed] [Google Scholar]
- 10.Viaggi S, Braselmann H, Nüsse M. Flow cytometric analysis of micronuclei in the CD2+ subpopulation of human lymphocytes enriched by magnetic separation. Int J Radiat Biol. 1995;67:193–202. doi: 10.1080/09553009514550241. [DOI] [PubMed] [Google Scholar]
- 11.Nüsse M, Marx K. Flow cytometric analysis of micronuclei in cell cultures and human lymphocytes: advantages and disadvantages. Mutat Res. 1997;392:109–115. doi: 10.1016/s0165-1218(97)00049-9. [DOI] [PubMed] [Google Scholar]
- 12.Avlasevich SL, Bryce SM, Cairns SE, Dertinger SD. In vitro micronucleus scoring by flow cytometry: differential staining of micronuclei versus apoptotic and necrotic chromatin enhances assay reliability. Environ Molec Mutagen. 2006;47:56–66. doi: 10.1002/em.20170. [DOI] [PubMed] [Google Scholar]
- 13.Bryce SM, Bemis JC, Avlasevich SL, Dertinger SD. In vitro micronucleus assay scored by flow cytometry provides a comprehensive evaluation of cytogenetic damage and cytotoxicity. Mutat Res. 2007;630:78–91. doi: 10.1016/j.mrgentox.2007.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Greenwood SK, Hill RB, Sun JT, Armstrong MJ, Johnson TE, Gara JP, Galloway SM. Population Doubling: A simple and more accurate estimation of cell growth suppression in the in vitro assay for chromosomal aberrations that reduces irrelevant positive results. Environ Molec Mutagen. 2004;43:36–44. doi: 10.1002/em.10207. [DOI] [PubMed] [Google Scholar]
- 15.Fenech M, Chang WP, Kirsch-Volders M, Holland N, Bonassi S, Zeiger E. HUMN project: detailed description of the scoring criteria for the cytokinesis block micronucleus assay using isolated human lymphocyte cultures. Mutat Res. 2003;534:65–75. doi: 10.1016/s1383-5718(02)00249-8. [DOI] [PubMed] [Google Scholar]
- 16.Meintieres S, Biola A, Pallardy M, Marzin D. Apoptosis can be a confusing factor in in vitro clastogenic assays. Mutagenesis. 2001;16:243–250. doi: 10.1093/mutage/16.3.243. [DOI] [PubMed] [Google Scholar]
- 17.Meintieres S, Biola A, Pallardy M, Marzin D. Using CTLL-2 and CTLL-2 bcl2 cells to avoid interference by apoptosis in the in vitro micronucleus test. Environ Molec Mutagen. 2003;41:14–27. doi: 10.1002/em.10126. [DOI] [PubMed] [Google Scholar]
- 18.Kirkland D, Aardema M, Henderson L, Müller L. Evaluation of the ability of a battery of three in vitro genotoxicity tests to discriminate rodent carcinogens and non-carcinogens. I. Sensitivity, specificity and relative predictivity. Mutat Res. 2005;584:1–256. doi: 10.1016/j.mrgentox.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 19.Kirkland D, Pfuhler S, Tweats D, Aardema M, Corvi R, Darroudi F, Elhajouji A, Glatt H, Hastwell P, Hayashi M, Kasper P, Kirchner S, Lynch A, Marzin D, Maurici D, Meunier JR, Müller L, Nohynek G, Parry J, Parry E, Thybaud V, Tice R, van Benthem J, Vanparys P, White P. How to reduce false positive results when undertaking in vitro genotoxicity testing and thus avoid unnecessary follow-up animal tests: Report of an ECVAM Workshop. Mutat Res. 2007;628:31–55. doi: 10.1016/j.mrgentox.2006.11.008. [DOI] [PubMed] [Google Scholar]
- 20.Hilliard CA, Armstrong MJ, Bradt CI, Hill RB, Greenwood SK, Galloway SM. Chromosome aberrations in vitro related to cytotoxicity of nonmutagenic chemicals and metabolic poisons. Environ Molec Mutagen. 1998;31:316–326. [PubMed] [Google Scholar]
- 21.Galloway SM. Cytotoxicity and chromosome aberrations in vitro: experience in industry and the case for an upper limit on toxicity in the aberration assay. Environ Molec Mutagen. 2000;35:191–201. [PubMed] [Google Scholar]
- 22.Fellows MD, O’Donovan MR. Cytotoxicity in cultured mammalian cells is a function of the method used to estimate it. Mutagenesis. 2007;22:275–280. doi: 10.1093/mutage/gem013. [DOI] [PubMed] [Google Scholar]
- 23.Collins JE, Ellis PC, White AT, Booth AEG, Moore CE, Burman M, Rees RW, Lynch AM. Evaluation of the Litron In Vitro MicroFlow® Kit for the Flow Cytometric Enumeration of Micronuclei (MN) in Mammalian Cells. doi: 10.1016/j.mrgentox.2008.05.003. manuscript in preparation. [DOI] [PubMed] [Google Scholar]
- 24.Matsuoka A, Yamazaki N, Suzuki T, Hayashi M, Sofuni T. Evaluation of the micronucleus test using a Chinese hamster cell line as an alternative to the conventional in vitro chromosomal aberration test. Mutat Res. 1993;272:223–236. doi: 10.1016/0165-1161(92)91535-y. [DOI] [PubMed] [Google Scholar]
- 25.Garriott ML, Phelps JB, Hoffman WP. A protocol for the in vitro micronucleus test. I. Contributions to the development of a protocol suitable for regulatory submissions from an examination of 16 chemicals with different mechanisms of action and different levels of activity. Mutat Res. 2002;517:123–134. doi: 10.1016/s1383-5718(02)00059-1. [DOI] [PubMed] [Google Scholar]
- 26.Phelps JB, Garriott ML, Hoffman WP. A protocol for the in vitro micronucleus test. II. Contributions to the validation of a protocol suitable for regulatory submissions from an examination of 10 chemicals with different mechanisms of action and different levels of activity. Mutat Res. 2002;521:103–112. doi: 10.1016/s1383-5718(02)00221-8. [DOI] [PubMed] [Google Scholar]
- 27.von der Hude W, Kalweit S, Engelhardt G, McKiernan S, Kasper P, Slacik-Erben R, Miltenburger HG, Honarvar N, Fahrig R, Gorlitz B, Albertini S, Kirchner S, Utesch D, Potter-Locher F, Stopper H, Madle S. In vitro micronucleus assay with Chinese hamster V79 cells—results of a collaborative study with in situ exposure to 26 chemical substances. Mutat Res. 2000;468:137–163. doi: 10.1016/s1383-5718(00)00045-0. [DOI] [PubMed] [Google Scholar]
- 28.Knasmüller S, Mersch-Sundermann V, Kevekordes S, Darroudi F, Huber WW, Hoelzl C, Bichler J, Majer BJ. Use of human-derived liver cell lines for the detection of environmental and dietary genotoxicants: current state of knowledge. Toxicology. 2004;198:315–328. doi: 10.1016/j.tox.2004.02.008. [DOI] [PubMed] [Google Scholar]
- 29.Zhan L, Sakamoto H, Sakurab M, Wu S, Zhang LS, Suzuki T, Hayashi M, Honma M. Genotoxicity of microcystin-LR in human lymphoblastoid TK6 cells. Mutat Res. 2004;557:1–6. [PubMed] [Google Scholar]
- 30.Hastwell PW, Chai LL, Roberts KJ, Webster TW, Harvey JS, Rees RW, Walmsley RM. High-specificity and high-sensitivity genotoxicity assessment in a human cell line: Validation of the GreenScreen HC GADD45a-GFP genotoxicity assay. Mutat Res. 2006;607:160–175. doi: 10.1016/j.mrgentox.2006.04.011. [DOI] [PubMed] [Google Scholar]