Abstract
During the late stages of Schistosoma mansoni infection, adult schistosomes catabolize host erythrocytic hemoglobin. In order to evade the toxic effects of free heme, the blood fluke biomineralizes dimeric heme into an inert crystalline pigment called hemozoin. In the present study, the chemical reactivity of schistosomal hemozoin (SmHz) toward lipid oxidation was examined and the biological consequences of reactivity were investigated. Mass spectrometric analysis of polar lipid content associated with SmHz identified a variety of primary and secondary polyunsaturated fatty acid oxidation products, including hydroxyeicosatetraenoic acids. Furthermore, RAW 264.7 macrophage-like cells challenged with lipopolysaccharide prior to phagocytosis of SmHz experienced a decrease in nitric oxide production as compared to control experiments. The presence of these biologically active oxidation products suggests native SmHz is capable of modulating the innate immune response and may play a potential role in the pathogenesis of schistosomiasis.
Keywords: Hemozoin, Schistosoma mansoni, Hydroxyeicosatetraenoic acid, Eicosanoid
Introduction
Schistosomiasis is a parasitic disease that chronically infects over 200 million people in 74 countries and is responsible for more than 200,000 deaths each year [1–3]. Based on recent reports, schistosomiasis ranks second only to malaria as a cause for chronic morbidity among tropical diseases [4]. Parasitic infection by Schistosoma mansoni occurs via a complex digenetic life cycle. In order to obtain requisite amino acids for growth and development, the blood fluke catabolizes host hemoglobin (Hb) from red blood cells (RBCs). Adult female schistosomes consume an estimated 330,000 RBCs per hour [5] Erythrocytic Hb is broken down by proteolytic enzymes in the gut releasing significant quantities of potentially toxic free heme [6]. To avoid these cytotoxic effects [7, 8], blood feeding organisms have developed novel mechanisms for heme detoxification.
In the protozoan P. falciparum, toxic free heme accumulates in the digestive food vacuole and is sequestered by aggregation into an inert crystalline pigment termed hemozoin (Hz) [9]. S. mansoni appears to employ a similar heme detoxification pathway. During Hb catabolism in the digestive cavity of the trematode, free heme aggregates, and the subsequent pigment is regurgitated by the parasite into the surrounding host vasculature. This dark-brown pigment was recently characterized by Oliveira and coworkers as being structurally identical to P. falciparum Hz (PfHz) and its synthetic analogue, β-hematin (BH) [10, 11].
Hz has long been considered an inert, detoxification end-product, but recent studies suggest that native PfHz is capable of modulating host immunity. Schwarzer and coworkers showed that phagocytosis of native PfHz by human monocytes disrupts the normal cellular function of professional phagocytes [12, 13]. The accumulation of native Hz in circulating monocytes and neutrophils inhibits normal phagocytic function by inducing oxidative stress and down-regulating intercellular mechanisms that generate potent oxidative molecules used to defend against foreign invaders, namely reactive oxygen (ROS) and nitrogen species (RNS) [14]. Furthermore, Hz has been shown to induce lipid peroxidation of polyunsaturated fatty acids (PUFAs) resulting in the formation of potentially cytotoxic and immunomodulatory molecules including hydroxyeicosatetraenoic acids (HETEs) [7, 13, 15] and 4-hydroxy-2-nonenal (HNE) [16, 17]. Consequently, Hz-mediated production of arachidonic acid (AA) metabolites, such as HETEs and HNE, are of significant interest in schistosomiasis since the role of Hz in the disease’s pathogenesis remains largely unexplored.
Materials and Methods
Materials
S. mansoni infected Swiss-Webster mice were provided courtesy of Dr. Fred Lewis and the NIAID Schistosomiasis Resource Center (Rockville, MD). Hydroxyeicosatetraenoic acid standards (Cayman Chemical Company, Ann Arbor, MI). RPMI 1640 (Cellgro Mediatech, Inc., Herdon, VA). Arachidonic acid (Nu-Chek Prep Inc., Elysian, MN). 4-hydroxy-2-nonenal (Calbiochem, San Diego, CA). Lipopolysaccharide (E. coli 055:B5), chelex, sulfanilamide, N-(1-naphthyl)ethylenediamine, 2,6-di-tert-butyl-4-methylphenol (BHT) and hemin (Fluka-brand, >98% purity) (Sigma-Aldrich, St. Louis, MO).
β-hematin synthesis
BH was synthesized via dehydrohalogenation of hemin, as described by Bohle [18]. The reaction vessel was sealed, protected from light, and stood undisturbed for 3 months. The resulting mixture was filtered, and the precipitate was washed exhaustively with methanol, 0.1 M sodium bicarbonate (pH 9.1) and deionized water. The purified product was dried under vacuum at 150°C for 48 hr and stored under dessicant.
SmHz Isolation
Adult schistosomes were obtained by mesenteric perfusion of mice 42 days post-infection [19]. Cultivated adult female worms were homogenized in phosphate buffered saline (PBS, pH 7.4) using a 15 mL glass homogenizer. Homogenate was centrifuged twice at 1000 × g for 60 s, and the tissue pellet was discarded. The supernatant was centrifuged 2 h at 5000 × g. The resulting dark-brown pellet was resuspended in 5 mL sterile PBS after vortexing and gentle sonication for 5 minutes. SmHz recovery was quantified as described by Sullivan et al. [20] with 20 mM NaOH, 2 % SDS at 25°C for 2 h. Heme content was determined from absorbance at 400 nm (ε = 1 × 105 M−1cm−1) using an Agilent 8453 UV-Visible Spectrophotometer.
Native SmHz Lipid Extraction
The native SmHz lipid component was extracted twice from 2.5 mg/mL native SmHz in 25 mM chelexed-phosphate buffer (pH 7.4) using 1:1 (v/v) ethyl acetate. The emulsion was vortexed 60 s and centrifuged 30 min. 5500 × g. The organic layer was removed and evaporated under nitrogen. The lipids were reconstituted in 1 mL of ethanol and stored at −80 °C.
In vitro Hz-Mediated HETE Production
Purified native SmHz, complete removal of lipids confirmed by NP-HPLC (data not shown), was used in these experiments. The reaction was performed by adding 5.32 mM AA to a 2 mL suspension of either SmHz or BH (0.3 mg/mL) in 25 mM chelexed-phosphate buffer (pH 7.4) for 4 hr at 25°C. Reaction products were extracted with ethyl acetate and stored in ethanol at −80°C.
LC-MS/MS HETE Identification
HETE standards and lipid samples were dried under nitrogen and resuspended in acetonitrile/water (30:70), 10 mM ammonium carbonate and 0.1 M BHT prior to injection. The LC-MS/MS analysis was performed on a Waters Acquity UPLC system (Waters, Milford, MA) connected to a Thermo Finnigan LTQ linear ion trap mass spectrometer (Thermo Fisher Scientific, Waltham, MA) using a Thermo Hypersil Gold C18 column (1.9 μm, 2.1 mm × 150 mm). LC conditions were as follows: solvent A contained 10 mM ammonium carbonate in acetonitrile/water (10:90) and solvent B contained 10 mM ammonium carbonate in acetonitrile/water (90:10). The following gradient program was used with a flow rate of 600 μL/min: 0–0.25 min, 30% B; 0.25–9 min, linear gradient 30–45%; 9–10 min, 45% B; 10–10.50 min, linear gradient 45–30% B; 10.50–17 min, 30%B. Sample injection volume was 10 μL. The following optimized parameters were used for the detection of analyte and internal standard: N2 sheath gas 36 psi; N2 auxiliary gas 20 psi; capillary temperature 300 C; source voltage 3.8 kV; source current 100 μA; skimmer offset 0.00 V; capillary offset −44.00 V; tube lens offset −103.30 V; activation time 30 ms (MS), 50 ms (MS2); isolation width 1 m/z (MS), 2 m/z (MS2). Data acquisition and quantitative spectral analysis was conducted using the Thermo-Finnigan Xcaliber software, version 2.0 Sur 1.
LC-MS/MS Chiral Separation
NP-LC
Normal Phase LC analysis was performed prior to chiral RP-LC-MS/MS in order to isolate HETE isomers for individual analysis. The NP-LC was performed on a Waters 600 HPLC connected to a Waters 996 photodiode array (Waters, Milford, MA) using a Beckman Ultrasphere silica column (5 μm, 4.6 mm × 25 cm; Beckman Coulter, Fullerton, CA). An isocratic gradient of 98.8% hexane, 1.2% isopropanol, and 0.1% acetic acid was used at a flow rate of 1.00 mL/min. HETE absorbance was detected at 237 nm.
Chiral-RP-LC- MS/MS
The LC-MS/MS analysis was performed as before using a CHIRALPAK AD-RH column (5 μm, 2.1 mm × 150 mm; Chiral Technologies, Inc., West Chester, PA). LC conditions were as follows: solvent A contained 0.25% formic acid in acetonitrile/water (5:95) and solvent B contained 0.25% formic acid in acetonitrile/water (95:5). The following gradient program was used with a flow rate of 200 μL/min: 0–5 min, 50% B; 5–20 min, linear gradient 50–100%; 20–25 min, 100% B; 25–30 min, linear gradient 100–50% B; 30–32 min, 50%B. The following optimized parameters were used for the detection of analyte and internal standard: N2 sheath gas 36 psi; N2 auxiliary gas 20 psi; capillary temperature 300 C; source voltage 3.8 kV; source current 100 μA; skimmer offset 0.00 V; capillary offset −44.00 V; tube lens offset −103.30 V; activation time 30 ms (MS), 50 ms (MS2); isolation width 1 m/z (MS), 2 m/z (MS2). Data acquisition and quantitative spectral analysis were conducted using Thermo-Finnigan Xcaliber version 2.0 Sur 1.
iNOS Inhibition
Effects of 5-, 12-, 15-S-HETE, HNE, and SmHz on LPS-stimulated inducible nitric oxide synthase (iNOS) activity in RAW 264.7 cells (1 × 105 cells/well in 96-well culture plates at 37°C in 5% CO2 for ≥8 h) was examined. Cell viability was confirmed using Trypan Blue exclusion. Stock solutions of 5-, 12-, and 15-S-HETE were prepared by evaporating each 100 μg vial to near dryness under nitrogen and reconstituting in RPMI complete medium (ethanol concentrations below 0.5%). Additionally, 0.1 mg/mL stock solutions of native SmHz, BH and 17.5 μM HNE were prepared in RPMI complete medium. Following stock solution treatment in the presence of LPS (1 μg/mL), cell cultures were incubated at 37°C in 5% CO2 for 24 h. The Griess reaction was used to measure nitrite concentration [21]. Absorbance of the resultant azo complex was measured at 540 nm using a Bio-Tek Synergy HT Multidetection Microplate Reader.
SEM
Dry purified SmHz was suspended in ethanol, sonicated for 10 min., applied to a polished aluminum specimen mount and dried at 25°C overnight. Each sample was sputter-coated with gold for 20 s and imaged using a Hitachi S4200 scanning electron microscope at 5.0 kV accelerating voltage.
Results and Discussion
Schistosomal pigment (SmHz) is typically found accumulated in resident tissue macrophages of the liver. Adult worm pairs reside in the hepatic portal system of the host and regurgitate hemozoin and cellular debris into the surrounding vasculature. Adult female worms exhibit a much heavier degree of pigmentation relative to males due to the nutritional demands of oogenesis (Fig. 1A). Following perfusion of the hepatic portal vein in S. mansoni-infected Swiss Webster mice, adult female and male worms were separated, counted and homogenized to determine SmHz burden per worm as previously described [20]. On average, adult females were found to accumulate 1.729 ± 0.29 μg of hemozoin while the adult males were found to amass only 0.114 ± 0.07 μg per worm. Isolated SmHz was purified and characterized using X-ray powder diffraction (characteristic 2:1 intensity of the signature 2θ peaks at 7° :21° and 24°) and Fourier transform infrared spectroscopy (1664 cm−1 and 1211 cm−1 of C=O and C-O stretching) confirming a dimeric ferriprotoporphyrin IX aggregate identical to PfHz and BH (SI 1). While PfHz and BH SEM images portray a biomineral of elongated rectangular crystals with well-defined crystal facets, SmHz images depict the characteristic heterogeneous morphology and large size distribution often attributed to the extracellular environment of Hz formation in S. mansoni (Fig. 1B) [11].
Figure 1.
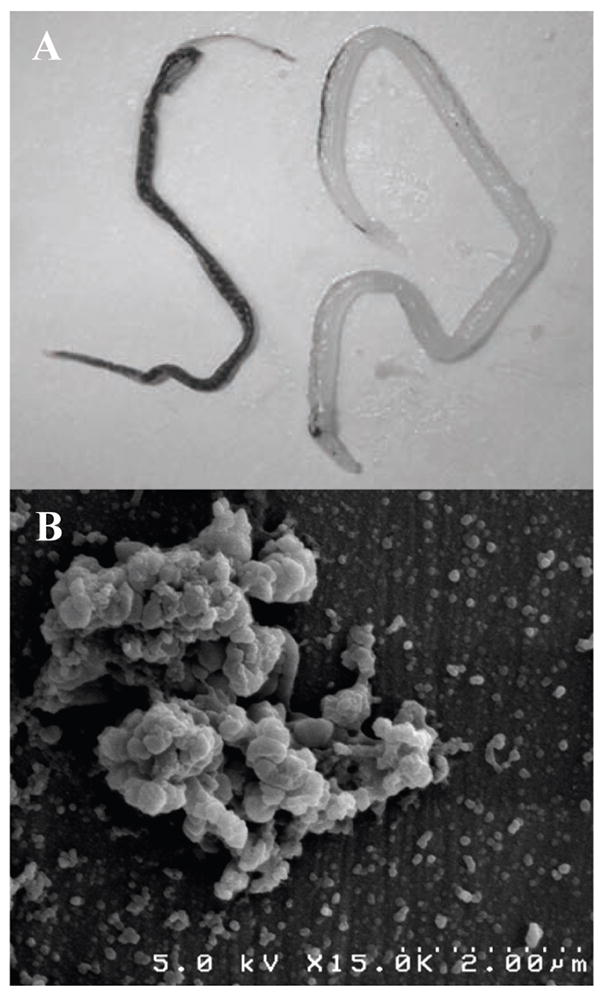
Hemozoin from S. mansoni. (A) Adult female (left) and male (right) S. mansoni laden with hemozoin. Female worms accumulate a heavier hemozoin burden, as seen by their darker pigmentation, due to the nutritional demands of oogenesis. (B) SEM image of schistosomal hemozoin isolated from homogenized S. mansoni.
The nature and function of the lipid environment surrounding the heme aggregate of native Hz is complex. In a recent study, transmission electron microscopy (TEM) showed the localization of SmHz at the hydrophilic-hydrophobic interface of lipid droplets in the S. mansoni gut lumen [22]. Similarly, Pisciotta and coworkers extracted a suite of neutral lipids coating native Hz from the digestive food vacuole of P. falciparum [23]. In both cases, the lipid extracts provided a competent scaffold for hemozoin formation in vitro [22, 23]. Polar hydroxylated fatty acids derived from arachidonic and linoleic acids have also been extracted from native PfHz, as has the secondary oxidation product HNE [7, 13, 17]. These compounds have been shown to be capable of significantly altering the function of macrophage cells during the course of malarial infection.
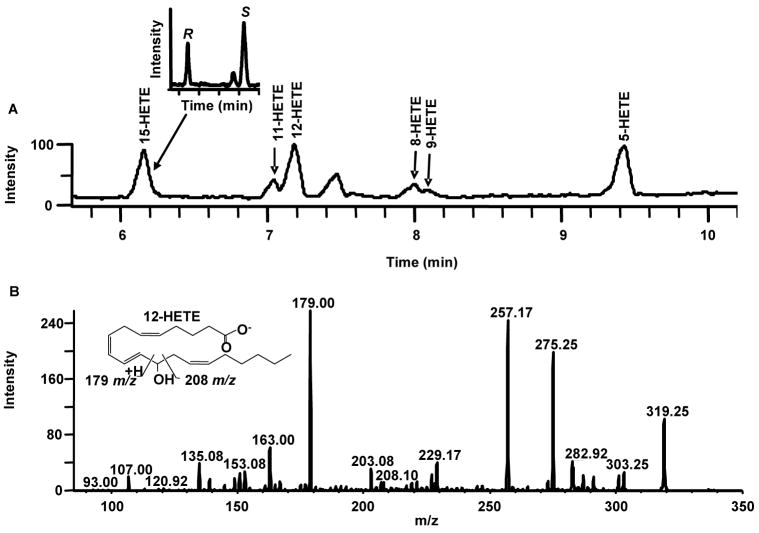
To investigate the presence of biologically active AA metabolites in native SmHz, the hydroxylated fatty acid component of native SmHz was isolated and analyzed by liquid chromatography-tandem mass spectrometry (LC-MS/MS). The identification of each HETE positional isomer peak was achieved using time dependent MS/MS in which the isobaric carboxylate [M-H]−, 319 m/z, precursor ion was trapped and subsequently fragmented to give product ions of each HETE isomer (SI 2). All six HETE positional isomers derived from AA were observed in the SmHz lipid extract, with the most abundant species being 15-, 12- and 5-HETE ([M-ROH]−: 219, 179 and 115 m/z, respectively) (Fig. 2). In contrast to previous HETE identifications in hemozoin producing parasites [13], the LC-MS/MS method presented herein is a marked advancement including MS/MS confirmation of all identified HETEs during separation and an increased instrument sensitivity reaching the femtomole range. Such a limit of detection is especially important when working with the extracted lipids of limited native Hz samples, as is the case of S. mansoni yields from Swiss-Webster mice (1 mg SmHz/60 mice).
Figure 2.
HETE-containing lipid coat of native SmHz. (A) Lipids were extracted from native SmHz using ethyl acetate, blown dry under N2 (g) and redissolved in RP-LC-MS/MS mobile phase. All six hydroxylated fatty acid derivatives of arachidonic acid were identified (15-, 11-, 12-, 8-, 9- and 5-HETE). Insert shows the representative chiral resolution for 15-HETE (11.5 min. R and 14.5 min. S). (B) Representative MS/MS fragmentation for 12-HETE. All isomers were confirmed using authentic standards and extracted ion profiles from MS/MS (SI 2).
Chiral purity of biologically derived hydroxylated fatty acids is typically attributed to the lipoxygenase (Lox) family [24–31] with preference usually shown for S-stereoisomer products. In the absence of Lox, however, free-radical mediated peroxidation of AA can yield a racemic mixture of each hydroxylated isomer. Given the presence of all six HETE isomers in the SmHz lipid coat, additional chiral analysis was performed to examine the enantiomeric distribution of the monohydroxy derivatives. Chiral LC-MS/MS of the SmHz lipid extract revealed an equivalent stereoselective distribution of all HETEs with the exception of 12-S-HETE (Fig. 2) (SI 3). Based on integrated peak area, a 70:1 abundance of 12-S-HETE to 12-R-HETE was observed suggesting accumulation of enzymatically produced HETE. This is in marked contrast to the equivalent stereoisomeric ratios observed for the 12-HETEs in the lipid coat of PfHz [13]. Previously, murine host 12-Lox activity has been described in the immunomodulation of schistosomiasis [32, 33]. Three types of murine 12-Lox (epidermal-, leukocyte- and platelet-type 12-Lox) have been reported, but none of these were observed in a recent proteomic analysis of gut contents from S. mansoni [32, 34]. In S. mansoni, only the activity of a 15-S-Lox has been reported, although sequence analysis of the partially sequenced S. mansoni genome [35] suggests the possible presence of a 12-S-Lox gene (data not shown). Regardless of the origin of the observed 12-S-HETE, the racemic mixture of all other HETE isomers is indicative of Hz-mediated lipid peroxidation of fatty acids.
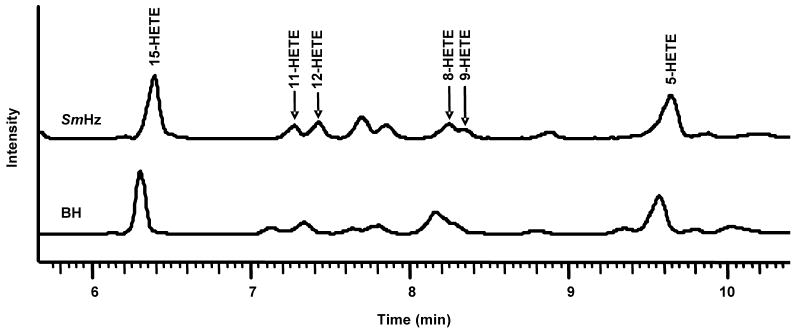
In order to confirm the expected Hz-driven lipid peroxidation of fatty acids, in vitro, reactions of AA incubated with either purified SmHz or BH were performed under aerobic conditions. The resultant oxidized lipids were extracted and characterized by RP-and chiral-LC-MS/MS. Relative to AA controls, both purified SmHz and BH produced significant quantities of the six HETE positional hydroxylation isomers, with 15- and 5-HETE as the most prominent (Fig. 3). These findings are in good agreement with earlier literature concerning the reactivity of both purified PfHz and BH [7, 36]. Upon subsequent chiral analysis, the positional HETE isomers were separated with a stereoselective ratio of nearly 1, suggesting no preferential stereochemical orientation of the substrate during oxidation. As expected, the stereoselective bias for 12-S-HETE seen in the native SmHz lipid coat was absent in the reaction of either purified SmHz or BH with AA. Taken together, these results demonstrate that SmHz mediated peroxidation of PUFA is the likely source for the enatiomeric population of observed HETE isomers in the lipid coat.
Figure 3.
Hemozoin-mediated lipid peroxidation. Oxidation studies by incubation of 0.3 mg/mL BH or SmHz with 5.32 mM AA were conducted. The hydroxylated fatty acid isomers 15-, 11-, 12-, 8-, 9- and 5-HETE were identified in the reactions’ lipid extracts using RP-LC-MS/MS. The isomers were confirmed using authentic standards and extracted ion profiles from MS/MS.
Lipid metabolites have been shown to elicit immunomodulatory effects ranging from impairment of both PMA-stimulated oxidative burst and nitric oxide (NO) production in macrophages to altering endothelial cell permeability and initiating chemotaxis [13, 37, 38]. Investigation of the HETE isomers’ individual effects on macrophage-like cell function following LPS challenge revealed that the predominate 5-, 12- and 15-S-HETEs impaired NO production in a dose-dependent manner (Fig. 4A). 12-S-HETE was the most potent inhibitor of iNOS, attenuating NO production by 50%. The immunosuppressive activity of native SmHz’s hydroxylated fatty acids suggest that phagocytosis of native SmHz by resident macrophages may partially disrupt the innate immune response. In fact, further study revealed a 25% reduction in NO production in lipid-coated SmHz-laden macrophages relative to control experiments, implicating native SmHz as a biologically active agent (Fig. 4B).
Figure 4.
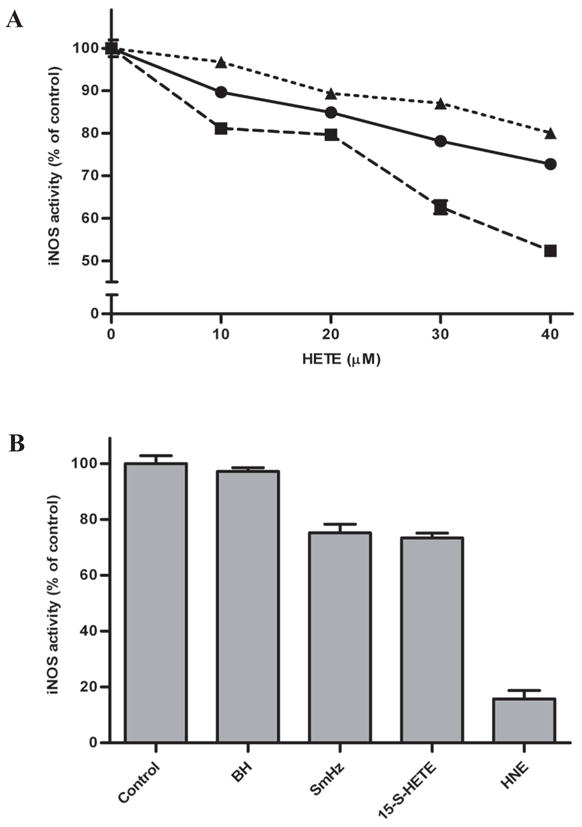
Impact of native SmHz and components on iNOS activity. (A) RNS production in macrophage cells monitored following treatment with increasing concentrations of (●) 15-, (■) 12- and (▲) 5-S-HETE by the Griess assay. (B) Cells were treated with 0.1 mg/mL BH or SmHz, as well as 40 μM 15-S-HETE or HNE. The activity of cells stimulated with LPS was used as a control.
The lipid coat of native SmHz is a complex mixture of both neutral lipids and PUFA peroxidation products. Previous analysis of the neutral lipids revealed the presence of monopalmitic-, monostearic-, dipalmitic-, dioleic- and dilinoleic glycerols [23]. Herein, the composition of the arachidonate metabolites has been determined to consist of an enantiomeric mixture of all positional hydroxylated isomers with 15-, 12-and 5-HETE as the dominant species. These findings are consistent with Hz-mediated lipid peroxidation of host arachidonate. Additional chiral analysis revealed an accumulation of a biologically derived 12-S-HETE of an unknown origin. When the immunosuppressive activity of native SmHz and its major HETE components was examined in macrophage-like cells, iNOS activity was impaired. Further exploration of this unique biomineral’s composition and its ability to modulate host immunity is crucial in developing a better understanding of the possible role of hemozoin in the host-pathogen interactions in S. mansoni.
Supplementary Material
SI 1. IR and XRD of Hz. (A) The presence of axial propionate linkages in Hz’s dimeric ferriprotoporphyrin IX structure was confirmed using infrared spectroscopy showing the C-O stretching at 1211 cm−1 and C=O stretching at 1664 cm−1. (B) Characterization by x-ray diffraction reveals the 2:1 intensity of 2? peaks at 7°: 21° and 24°. These peaks are absent in the substrate hemin chloride. Reduced peak distinction compared to BH in SmHz pattern is attributed to limited sample size.
SI 2. Extracted Ion Profile of SmHz Lipid Coat. The identification of all six positional isomers of HETE in the SmHz lipid coat was achieved by extracting the characteristic MS/MS fragment masses of each isomer from the collected 319 m/z reverse-phase-LC-MS ions. Individual isomers from the total ion chromatogram (A) of the SmHz lipid coat are shown: (B) 15-HETE at 6.16 min., (C) 11-HETE at 7.04 min., (D) 12-HETE at 7.19 min., (E) 8-HETE at 7.99 min., (F) 9-HETE at 8.10 min. and (G) 5-HETE at 9.42 min. Characteristic MS/MS fragments were confirmed using authentic HETE standards.
SI 3. Chiral-RP-LC-MS/MS of SmHz Lipid Coat. R and S HETE enantiomers were separated using chiral-reverse-phase-LC-MS/MS (R- and S-HETE retention times shown in figure key). Each HETE isomer was identified by its characteristic MS/MS fragment masses as determined from authentic HETE standards. A 1:1 ratio was observed for all HETE enantiomers with the exception of 12-HETE which exhibited a 70:1 distribution of 12-S-HETE to 12-R-HETE.
Acknowledgments
Financial support for this work was provided by the National Institutes of Health (R03AI060827 and U54AI057157). The authors would also like to thank Dr. David L. Hachey and the staff of the Vanderbilt University Mass Spectrometry Research Center, Dr. Fred A. Lewis and the staff of the NIAID Schistosomiasis Resource Center (NIAID Contract N01-A1-30026) and Dr. Elizabeth L. Bentzen and Jonas W. Perez.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.McManus DP. The search for a vaccine against Schistosomiasis - A difficult path but an achievable goal. Immunol Rev. 1999;171:149–161. doi: 10.1111/j.1600-065x.1999.tb01346.x. [DOI] [PubMed] [Google Scholar]
- 2.Pearce EJ, MacDonald AS. The immunobiology of Schistosomiasis. Nat Rev. 2002;2:499–511. doi: 10.1038/nri843. [DOI] [PubMed] [Google Scholar]
- 3.(2002) in WHO Technical Report Series 912, Geneva.
- 4.van der Werf MJ, de Vlas SJ, Brooker S, Looman CWN, Nagelkerke NJD, Habbema JDF, Engels D. Quantification of clinical morbidity associated with schistosome infection in sub-Saharan Africa. Acta Trop. 2003;86:125–139. doi: 10.1016/s0001-706x(03)00029-9. [DOI] [PubMed] [Google Scholar]
- 5.Lawrence JD. The ingestion of red blood cells by Schistosoma mansoni. J Parasitol. 1973;59:60–63. [PubMed] [Google Scholar]
- 6.Brindley PJ, Kalinna BH, Dalton JP, Day SR, Wong JYM, Smythe ML, McManus DP. Proteolytic degradation of host hemoglobin by schistosomes. Mol Biochem Parasitol. 1997;89:1–9. doi: 10.1016/s0166-6851(97)00098-4. [DOI] [PubMed] [Google Scholar]
- 7.Green MD, Xiao L, Lal AA. Formation of hydroxyeicosatetraenoic acids from hemozoin-catalyzed oxidation of arachidonic acid. Mol Biochem Parasitol. 1996;83:183–188. doi: 10.1016/s0166-6851(96)02769-7. [DOI] [PubMed] [Google Scholar]
- 8.Chou AC, Fitch CD. Mechanism of hemolysis induced by ferriprotoporphyrin IX. J Clin Invest. 1981;68:672–677. doi: 10.1172/JCI110302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Francis SE, Sullivan DJ, Jr, Goldberg DE. Hemoglobin metabolism in the malaria parasite Plasmodium falciparum. Annu Rev Microbiol. 1997;51:97–123. doi: 10.1146/annurev.micro.51.1.97. [DOI] [PubMed] [Google Scholar]
- 10.Oliveira MF, d’Avila JCP, Torres CR, Oliveira PL, Tempone AJ, Rumjanek FD, Braga CMS, Silva JR, Dansa-Petretski M, Oliveira MA, Souza Wd, Ferreira ST. Haemozoin in Schistosoma mansoni. Mol Biochem Parasitol. 2000;111:217–221. doi: 10.1016/s0166-6851(00)00299-1. [DOI] [PubMed] [Google Scholar]
- 11.Oliveira MF, Kycia SW, Gomez A, Kosar AJ, Bohle DS, Hemplemann E, Menezes D, Vannier-Santos MA, Oliveira PL, Ferreira ST. Structural and morphological characterization of hemozoin produced by Schistosoma mansoni and Rhodnius prolixus. FEBS Lett. 2005;579:6010–6016. doi: 10.1016/j.febslet.2005.09.035. [DOI] [PubMed] [Google Scholar]
- 12.Schwarzer E, Turrini F, Ulliers D, Giribaldi G, Ginsburg H, Arese P. Impairment of macrophage functions after ingestions of Plasmodium falciparum-infected erythrocytes or isolated malarial pigment. J Exp Med. 1992;176 doi: 10.1084/jem.176.4.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schwarzer E, Kuhn H, Valente E, Arese P. Malaria-parasitized erythrocytes and hemozoin nonenzymatically generate large amounts of hydroxy fatty acids that inhibit monocyte functions. Blood. 2003;101:722–728. doi: 10.1182/blood-2002-03-0979. [DOI] [PubMed] [Google Scholar]
- 14.Urban BC, Roberts DJ. Malaria, monocytes, macrophages and myeloid dendritic cells: sticking of infected erythrocytes switches off host cells. Curr Opin Immunol. 2002;14:458–465. doi: 10.1016/s0952-7915(02)00368-0. [DOI] [PubMed] [Google Scholar]
- 15.Giribaldi G, Ulliers D, Schwarzer E, Roberts I, Piacibello W, Arese P. Hemozoin- and 4-Hydroxynonenal-mediated inhibition of erythropoiesis. Possible role in malarial dyserythropeoiesis and anemia. Haematologica. 2004;89:492–493. [PubMed] [Google Scholar]
- 16.Miller CM, Carney CK, Schrimpe AC, Wright DW. Beta-hematin (hemozoin) mediated decomposition of polyunsaturated fatty acids to 4-hydroxy-2-nonenal. Inorg Chem. 2005;44:2134–2136. doi: 10.1021/ic048821i. [DOI] [PubMed] [Google Scholar]
- 17.Schwarzer E, Muller O, Arese P, Siems WGW, Grune T. Increased levels of 4-hydroxynonenal in human monocytes fed with malarial pigment hemozoin: a possible clue for hemozoin toxicity. FEBS Lett. 1996;388:119–122. doi: 10.1016/0014-5793(96)00523-6. [DOI] [PubMed] [Google Scholar]
- 18.Bohle DS, Helms JB. Synthesis of beta-hematin by dehydrohalogenation of hemin. Biochem Biophys Res Commun. 1993;193:504–508. doi: 10.1006/bbrc.1993.1652. [DOI] [PubMed] [Google Scholar]
- 19.Lewis F. Schistosomiasis. John Wiley & Sons; 1998. pp. 19.11.11–19.11.28. [Google Scholar]
- 20.Sullivan DJ, Jr, Gluzman IY, Russell DG, Goldberg DE. On the molecular mechanism of chloroquine’s antimalarial action. Proc Natl Acad Sci USA. 1996;93:11865–11870. doi: 10.1073/pnas.93.21.11865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nagano T. Practical methods for detection of nitric oxide. Luminescence. 1999;14:283–290. doi: 10.1002/(SICI)1522-7243(199911/12)14:6<283::AID-BIO572>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 22.Soares JBRC, Maya-Monteiro CM, Bittencourt-Cunha PRB, Atella GC, Lara FA, d’Avila JCP, Menezes D, Vannier-Santos MA, Oliveira PL, Egan TJ, Oliveira MF. Extracellular lipid droplets promote hemozoin crystallization in the gut of the blood fluke Schistosoma mansoni. FEBS Lett. 2007;581:1742–1750. doi: 10.1016/j.febslet.2007.03.054. [DOI] [PubMed] [Google Scholar]
- 23.Pisciotta JM, Coppens I, Tripathi AK, Scholl PF, Shuman J, Bajad S, Shulaev V, Sullivan DJ., Jr The role of neutral lipid nanospheres in Plasmodium falciparum haem crystallization. Biochem J. 2007;402:197–204. doi: 10.1042/BJ20060986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hada T, Swift LL, Brash AR. Discovery of 5R-lipoxygenase activity in oocytes of the surf clam, Spisula solidissima. Biochim Biophys Acta. 1997;1346:109–119. doi: 10.1016/s0005-2760(96)00179-8. [DOI] [PubMed] [Google Scholar]
- 25.Jiang ZD, Gerwick WH. Novel oxylipins from the temperate red alga Polyneura latissima: evidence for an arachidonate 9(S)-lipoxygenase. Lipids. 1997;32:231–235. doi: 10.1007/s11745-997-0029-9. [DOI] [PubMed] [Google Scholar]
- 26.Jiang ZD, Ketchum SO, Gerwick WH. 5-Lipoxygenase-derived oxylipins from the red alga, Rhodymenia pertusa. Phytochemistry. 2000;53:129–133. doi: 10.1016/s0031-9422(99)00445-8. [DOI] [PubMed] [Google Scholar]
- 27.Hawkins DJ, Brash AR. Eggs of the sea urchin StrongylocentrotusI purpuratus, contain a prominent (11R) and (12R) lipoxygenase activity. J Biol Chem. 1987;262:7629–7634. [PubMed] [Google Scholar]
- 28.Coffa G, Hill EM. Discovery of an 11(R)- and 12(S)-lipoxygenase activity in ovaries of the mussel Mytilus edulis. Lipids. 2000;35:1195–1204. doi: 10.1007/s11745-000-0636-5. [DOI] [PubMed] [Google Scholar]
- 29.Boeglin WE, Kim RB, Brash AR. A 12R-lipoxygenase in human skin: mechanistic evidence, molecular cloning and expression. Proc Natl Acad Sci USA. 1998;95:6744–6749. doi: 10.1073/pnas.95.12.6744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yamamoto S, Suzuki H, Ueda N. Arachidonate 12-lipoxygenases. Prog Lipid Res. 1997;36:23–41. doi: 10.1016/s0163-7827(97)00002-7. [DOI] [PubMed] [Google Scholar]
- 31.Samuelsson B, Dahlen SE, Lindgren JA, Rouzer CA, Serhan CN. Leukotrienes and lipoxins: structures, biosynthesis, and biological effects. Science. 1987;237:1171–1176. doi: 10.1126/science.2820055. [DOI] [PubMed] [Google Scholar]
- 32.Burger F, Krieg P, Marks F, Furstenberger G. Positional- and stereo-selectivity of fatty acid oxygenation catalysed by mouse (12S)-lipoxygenase isoenzymes. Biochemical Journal. 2000;348:329–335. [PMC free article] [PubMed] [Google Scholar]
- 33.Secor WE, Powell MR, Morgan J, Wynn TA, Funk CD. Mice deficient for 5-lipoxygenase, but not leukocyte-type 12-lipoxygenase, display altered immune responses during infection with Schistosoma mansoni. Prostaglandins & Other Lipid Mediators. 1998;56:291–304. doi: 10.1016/s0090-6980(98)00059-8. [DOI] [PubMed] [Google Scholar]
- 34.Delcroix M, Medzihradsky K, Caffrey CR, Fetter RD, McKerrow JH. Proteomic analysis of adult S. mansoni gut contents. Mol Biochem Parasitol. 2007;154:95–97. doi: 10.1016/j.molbiopara.2007.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.in GeneDB, Sanger Institute.
- 36.Porter NA, Logan J, Kontoyiannidou V. Preparation and purification of arachidonic acid hydroperoxides of biological importance. J Org Chem. 1979;44:3177–3181. [Google Scholar]
- 37.Schwarzer E, Ludwig P, Valente E, Arese P. 15(S)-hydroxyeicosatetraenoic acid (15-HETE), a product of arachidonic acid peroxidation, is an active component of hemozoin toxicity to monocytes. Parassitologia. 1999;41:199–202. [PubMed] [Google Scholar]
- 38.Carney CK, Schrimpe AC, Halfpenny K, Harry RS, Miller CM, Broncel M, Sewell SL, Schaff JE, Deol R, Carter MD, Wright DW. The basis of the immunomodulatory activity of malaria pigment (hemozoin) J Biol Inorg Chem. 2006;11:917–929. doi: 10.1007/s00775-006-0147-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
SI 1. IR and XRD of Hz. (A) The presence of axial propionate linkages in Hz’s dimeric ferriprotoporphyrin IX structure was confirmed using infrared spectroscopy showing the C-O stretching at 1211 cm−1 and C=O stretching at 1664 cm−1. (B) Characterization by x-ray diffraction reveals the 2:1 intensity of 2? peaks at 7°: 21° and 24°. These peaks are absent in the substrate hemin chloride. Reduced peak distinction compared to BH in SmHz pattern is attributed to limited sample size.
SI 2. Extracted Ion Profile of SmHz Lipid Coat. The identification of all six positional isomers of HETE in the SmHz lipid coat was achieved by extracting the characteristic MS/MS fragment masses of each isomer from the collected 319 m/z reverse-phase-LC-MS ions. Individual isomers from the total ion chromatogram (A) of the SmHz lipid coat are shown: (B) 15-HETE at 6.16 min., (C) 11-HETE at 7.04 min., (D) 12-HETE at 7.19 min., (E) 8-HETE at 7.99 min., (F) 9-HETE at 8.10 min. and (G) 5-HETE at 9.42 min. Characteristic MS/MS fragments were confirmed using authentic HETE standards.
SI 3. Chiral-RP-LC-MS/MS of SmHz Lipid Coat. R and S HETE enantiomers were separated using chiral-reverse-phase-LC-MS/MS (R- and S-HETE retention times shown in figure key). Each HETE isomer was identified by its characteristic MS/MS fragment masses as determined from authentic HETE standards. A 1:1 ratio was observed for all HETE enantiomers with the exception of 12-HETE which exhibited a 70:1 distribution of 12-S-HETE to 12-R-HETE.