Figure 5. Ablation of peripheral dendritic cells in vivo markedly suppresses the CNS innate and adaptive antiviral immune response.
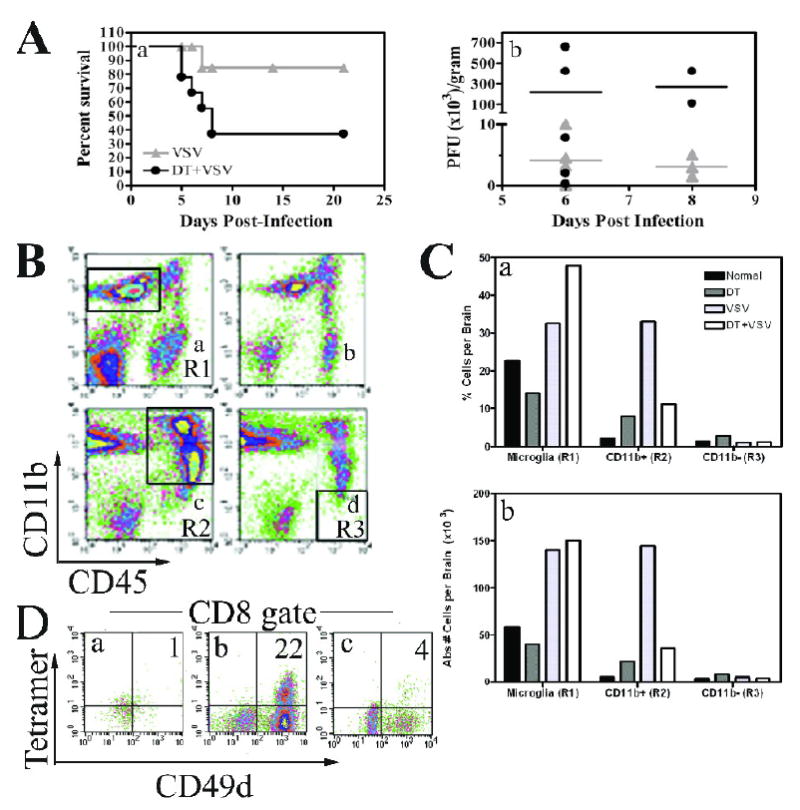
(A) DTRTg mice were given either PBS or DT one day before and after intranasal instillation of VSV (2×105 PFU). Mice were then monitored for morbidity (panel a). Mice were euthanized when moribund and brains and peripheral organs evaluated for VSV titres by plaque assay (panel b). This data is derived from 17 VSV-infected mice and 18 DT-treated, VSV-infected mice. (B) Mice were treated with either PBS (panels a, c) or DT (panels b, d). Cohorts either remained uninfected (panels a, b) or were given an intranasal inoculation of VSV at 2×105 PFU/mouse (panels c, d). Six days post-infection, brains were homogenized and then subjected to Percoll gradient centrifugation to enrich for leukocytes. Cells were then phenotyped by flow cytometry and a microglia gate defined as CD11b+CD45low/int cells (panel a, R1 gate). A second gate was established for peripheral mΦ/monocytes defined as CD11b+CD45high (panel c, R2). A final CD11b-CD45high gate was used to evaluate lymphocytes (panel d, R3). (C) The percent positive and absolute number of cells was then calculated within each of these gates and is summarized in the bar graphs. (D) To identify CD8+VSV-specific T cells, cells were first incubated with H-2Kb/VSV-N52-59 tetramers and then stained with mAbs to CD45, CD8, and the activation antigen CD49d. CD8+ cells were gated and the percentage of VSV-specific T cells within this gate determined by tetramer staining and co-expression of CD49d. Brains from 3-5 mice were pooled within each group. This experiment has been repeated two additional times and yielded similar results.
