Abstract
Cancer recurrence following response to therapy suggests that resistant cells lay dormant and subsequently reactivate. The cancer stem cell paradigm explains why tumors typically consist of a large therapy sensitive compartment, and a smaller compartment with profound intrinsic resistance. Here we examine co-expression of tissue stem cell markers (CD90, CD117, CD133) and cytokeratin in previously untreated non-small cell lung cancer (NSCLC). In normal lung (NL), we assign a provisional profile to resting stem cells (low scatter, cytokeratin- and either CD90dim/CD133+, or CD117+). Progenitors share this phenotype but are morphologically complex, downregulating CD90 as they gain cytokeratin. This pattern is retained in well-differentiated NSCLC, but is deranged in poorly-differentiated NSCLC, the most common pattern being overexpression of cytokeratin on stem/progenitors. Stem cells and progenitors are present at ~1% and 10% in NL and NSCLC, respectively. Constitutive MDR was present in ~6% of well-differentiated and ~50% of poorly differentiated tumors. We hypothesize that among the minority of tumor cells capable of propagating a tumor, only those that self-protect survive therapy. Of these, only cells which, like normal stem cells, are predominantly resting, cause recurrence after remission. The therapeutic index of antineoplastics becomes one of sensitivity of cancer and normal stem cells, which are protected by the same mechanisms.
Introduction
ABC transporters are highly conserved and represent a major protective mechanism for barrier tissues as well as adult tissue stem cells (1–9). Survival of tissue stem cells is essential to tissue maintenance and repair, and constitutive MDR activity is thought to be one of several protective mechanisms by which normal tissue stem cells guard themselves form from toxic insults, including those resulting from damage by chemotherapeutic agents (10). Moreover, in the absence of tissue stem cell specific markers, the activity of these transporters has been exploited to obtain enriched populations of tissue stem cells (5, 11, 12); the efflux or exclusion of fluorescent MDR substrates such as rhodamine 123 (ABCB1 substrate, R123dull/dim phenotype) and Hoechst33342 (ABCG2 and to a lesser degree ABCB1 substrate, Side Population (SP) phenotype) are frequently used in fluorescence activated cell sorting of hematopoietic and non-hematopoietic tissue stem cells.
The unique insight which we derive from the study of adult tissue stem cells and bone marrow derived stem cells (5,11) is that drug resistance, mediated in large part by ABC transporters, is a normal physiologic self-protective mechanism, which we hypothesize is retained by the nascent neoplasm upon transformation of the tissue stem cell (10). The notion that the cancer-initiating cell may have constitutive drug resistance predicts the persistence of a therapy resistant stem cell-like fraction following apparently successful cytotoxic cancer therapy resulting in the marked shrinkage or even disappearance of measurable tumor. Therapies that target proliferating cells, enzymes, growth receptors, adherence molecules, or signaling molecules in metabolically active cells may be highly effective at debulking tumors and dispatching tumorigenic cells without stem cell like properties, but they will consistently fail to eradicate the rare tumor cell fraction that shares protective mechanisms with their normal counterparts.
The cancer stem cell hypothesis attempts to explain the origin of cancer and identifies the cancer initiating cell as a transformed tissue stem cell. However, human cancers are heterogeneous and it is not always possible to apply a rigorous classification into stem, progenitor and mature compartments on the basis of differentiation marker expression and morphology. In normal adult tissues, stem cells are small resting undifferentiated cells which are confined to anatomic niches in which they are protected from toxic insults by both interaction with niche cells and by intrinsic mechanisms such as MDR transporter activity and detoxifying enzymes. When they are driven into proliferation, they replicate asymmetrically, giving rise to a stem cell daughter which remains in the niche, and a progenitor daughter (or transit amplifying cell) which migrates out of the niche, proliferates and efficiently gives rise to mature post mitotic cells with tissue specific characteristics. In doing so, the progenitor and its progeny progressively lose the markers of “stemness” (including self renewal and self-protection) as they gain tissue specific markers. However, there are variations on this theme, in which cells with mature function, such as hepatocytes, given appropriate stimuli, can revert to progenitor status and mediate tissue regeneration. It is also worthwhile to distinguish between three partially overlapping roles of tissue stem cells. The first role, organogenesis, is largely complete at birth, with the exception of the breast and prostate, which develop at puberty under hormonal influences. The second role, tissue maintenance, is a continuous process that proceeds at a rapid pace in some organs (skin, gut, blood) and at a glacial pace in others (nerves). Finally, adult tissue stem cells mediate tissue repair after injury, a function that is better developed in some organs (liver, blood) than in others (nerve). Whatever the role, the adult tissue stem cell is characterized by its anatomic location, and ability to self-replicate, self-protect, and give rise to further differentiated progeny of high proliferative capacity.
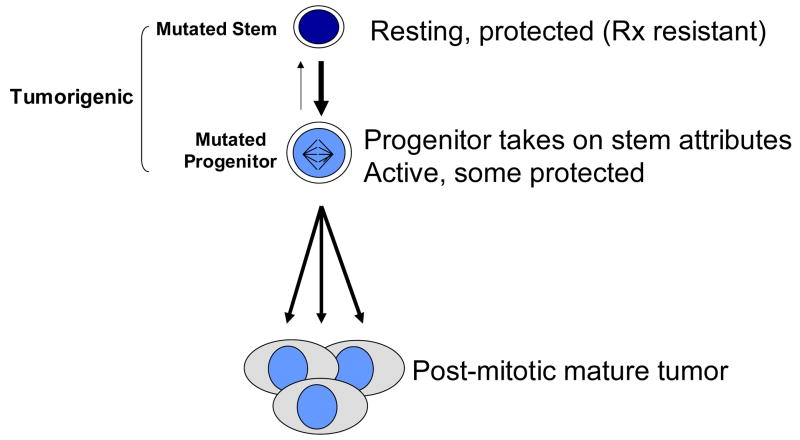
Returning to the cancer stem cell hypothesis, a strong analogy can be made between tumor growth and normal tissue growth in that: 1) The majority of tumor cells are post mitotic (non tumorigenic); 2) In order to be tumorigenic, at least a fraction of cells must be capable of sustained self-renewal (i.e. not loose proliferative capacity as a function of proliferative history); 3) In order to survive the toxic insults of therapy, a proportion of cells must retain or develop self-protective mechanisms (drug transport and metabolism). Figure 1 shows a schematic representation of the cancer stem cell hypothesis. A mutated stem cell is shown at the top of the chain, characterized by its resting protected state. These cells and their progeny, active, tumorigenic progenitor cells have already departed from the normal schema, as they retain the capacity for self renewal, as well as the ability to dedifferentiate into resting a resting protected phenotype. These last two points come from our experimental data showing that purified large, stem/progenitor marker positive (CD90), proliferating, MDR negative tumor cells are tumorigenic at very high frequency, and give rise to tumors as heterogeneous as those from which they were purified, including a small proportion of stem/progenitor marker+, MDR+ resting cells (13). In this report we will further explore the expression of stem and progenitor cell markers and MDR activity in lung cancer and normal lung tissue, with emphasis on reconciling the cancer stem cell paradigm with both low and high grade malignancies.
Figure 1. The cancer stem cell hypothesis attempts to explain the origin of cancer and identifies the cancer initiating cell as a transformed tissue stem cell.
However, human cancers are heterogeneous and it is not always possible to classify them into stem, progenitor and mature compartments on the basis of differentiation marker expression and morphology. That both stem and progenitor fractions are shown as tumorigenic is derived from our findings in breast cancer (13), as is the bidirectional path between stem and progenitor cells.
Methods
Samples and Sample Preparation
Single cell suspensions were obtained from normal lung (adjacent grossly normal tissue from lung cancer patients= 17) and malignant adult lung tissues (n = 20). Specimens were collected in accordance with a protocol approved by the University of Pittsburgh Internal Review Board. Tissues were minced with paired scalpels, digested with type I collagenase and disaggregated through 100 mesh stainless steel screens (4% in RPMI 1640 medium, Sigma Chemicals, St. Louis MO) (14). Ten to 500 million viable cells were recovered from a 5–10 mm3 specimens of tumor or normal lung parenchyma. Viable cells of single cell suspensions obtained from adjacent normal parenchyma, tumor and pleural effusions were concentrated and separated on a ficoll/hypaque gradient.
Staining and Flow Cytometry
Single cell suspensions were stained according to a protocol described in detail elsewhere (15). Five minutes prior to staining with fluorochrome-conjugated monoclonal antibodies, neat mouse serum (5 uL) was added to each cell pellet to minimize non-specific antibody binding. Prior to cytokeratin staining, cells were stained for surface markers (2 uL each added to the cell pellet, 15–30 min on ice), and fixed with 2% methanol-free formaldehyde. Cells were then permeabilized with 0.1% saponin (Beckman Coulter, Fullerton, CA) in phosphate buffered saline with 0.5% human serum albumin. Antibodies and dyes used in these studies included: Pan cytokeratin-FITC (Beckman Coulter, Cat. No. IM2356), CD90-biotin (BD, Cat. No. 555594), Streptavidin-ECD (Beckman Coulter, Cat. No. IM3326), ABCG2-PC5 (Chemicon, Temecula CA, Cat. No. MAB4155PC), CD117-PC7 (Beckman Coulter, Cat. No. IM3698), CD133-APC (Miltenyi Biotech, Cat. No. 120001241), HEA-APC (Miltenyi Biotech, Bergisch Gladbach, Germany, Cat. No. 12000420), CD45-APCC7 (BD, Cat. No. 557833), DAPI (Sigma Chemicals, St. Louis MO, Cat.), Propidium iodide (Calbiochem, La Jolla, CA, Cat. No. 537059), Rhodamine 123 (Sigma Chemicals, St. Louis MO, Cat. No. R8004), Hoechst 33342 (Invitrogen, Carlsbad, CA, Cat. No. H3570). Fumitremorgin was purchased from Alexis, (Cat. No. ALX-350-127). Eight-color analysis was performed using the 3-laser, 9-color CyAn LX cytometer (Dako, Fort Collins, CO). Analysis requiring an ultraviolet laser was performed on a 3-laser 8-color Dako MoFlo. An effort was made to acquire a total of 5–10 million cells per sample at rates not exceeding 10,000 events/second. The cytometers were calibrated prior to each use using SpectrAlign beads (Dako, Cat. No. KO111) and 8-peak Rainbow Calibration Particles (Spherotech, Libertyville, IL, Cat. No. RCP-30-5A). Color compensation matrices were calculated for each staining combination within each experiment using single-stained mouse IgG capture beads (Becton Dickinson, Cat. No. 552843) for each antibody, and single-stained cells for rhodamine 123. Offline analysis was performed using a prototype version of Venturi, a analytical package utilizing parallel processing and designed specifically for multiparameter rare event problems (Applied Cytometry Systems, Dinnington, Sheffield, UK). In all analyses, doublets and clusters were eliminated using forward scatter peak width versus height as a discriminator. Propidium iodide staining was used to eliminate nonviable cells, DAPI staining was used to eliminate hypodiploid cells. The channel normally used for PE (FL2, 575 nm) was used to identify and eliminate autofluorescent cells.
Statistics
Statistical analysis was performed using Systat version 11 (Systat Inc, San Jose, CA). Stem and progenitor cell frequencies were log normally distributed and were log transformed prior to analysis by ANOVA.
Results
Identification of candidate stem progenitor and mature populations in lung tumors and normal lung tissue
Cancer stem cells were initially defined by a combination of function and expression of a particular profile of adhesion molecules. In breast and pancreatic cancer, ESA+ CD44+ CD24- tumor cells were 100 to 200-fold enriched in cells that were tumorigenic in an explant model (16,17). Other investigators showed in human brain tumor that cells expressing CD133+, a marker originally described on hematopoietic progenitor cells, were enriched in tumorigenic cells (18). We have added CD90 to the list of markers on tumorigenic breast cancer cells (13). Strictly speaking, adult stem cells are normally resting cells which give rise to highly proliferative progenitor cells. In an attempt to shed light on the stem/progenitor distinction in cancer cells, we used 8-color flow cytometry to identify resting cells of low morphologic complexity (low light scatter), expressing the stem cell markers CD90, CD117 or CD133 in primary and metastatic lung cancer and in normal lung tissue. These are considered candidate cancer stem cells. Larger morphologically more complex cells expressing any of these same markers were considered candidates for cancer progenitor cells. Expression of intracellular cytokeratin was used to assess epithelial differentiation. All cells were gated to eliminate cell clusters, CD45+ hematopoietic cells, cells that had autofluorescence that spanned the wavelengths measured in FL1, FL2 and FL3 (525 – 620 nm), and DAPIdim cells with <2N DNA. Lymphocytes in the CD45+ fraction were used to define the light scatter properties of small resting cells. Three lung cancer pleural effusions, and 17 lung tumors with matched adjacent normal tissue were studied.
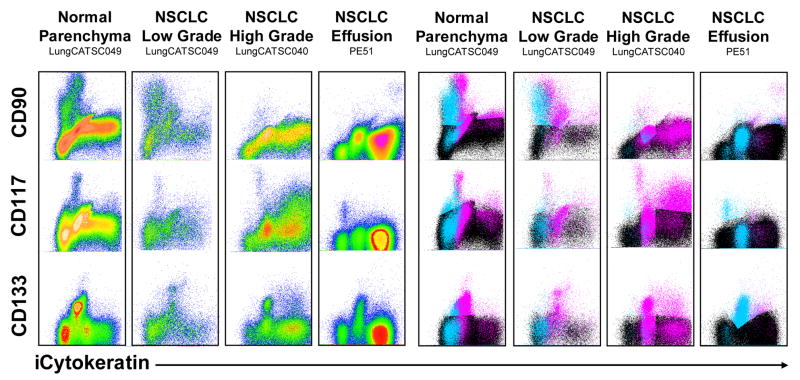
Figure 2 shows representative profiles from normal lung tissue adjacent to a resected tumor, a histologically well differentiated non-small cell adenocarcinoma of the lung, a poorly differentiated non-small cell adenocarcinoma of the lung, and a pleural effusion of metastatic non-small cell lung cancer. All samples were freshly isolated from newly diagnosed previously untreated patients. The left panels show density plots of the stem cell markers versus the epithelial differentiation marker intracellular cytokeratin; in the right panels, the same parameters are displayed as color event profiles, where the cyan dots are cells small resting cells expressing any combination of CD90, CD117, and CD133, magenta dots are large morphologically complex cells expressing one or more stem cell marker, and black dots are cells without any stem cell marker expression. Disaggregated normal lung is a complex tissue comprised of small airways, alveoli and small vessels; this complexity is reflected in the stem- progenitor profile. The small resting cells are virtually all cytokeratin negative, with a major population of CD90dim cells and minor populations of CD117+ and CD133+ cells. CD133+ and CD90dim are sometimes coexpressed (not shown), but CD117 is a unique population. The progenitor fraction is more heterogeneous, with CD90+ and CD133+ cells decreasing in fluorescence intensity as they mature from cytokeratin dim to bright. Even more so than the resting cells, CD90dim and CD133 expression is well correlated, whereas CD117 is present on a unique population. Despite the obvious gross differences between tumor and adjacent lung tissue, the well differentiated (low grade) non small cell lung cancer shows a stem-progenitor profile virtually identical to the normal lung tissue, which was isolated from the same patient. The relationship between CD90 and cytokeratin expression is particularly interesting, showing four discrete populations evocative of a differentiation pattern. Small resting cells are virtually all CD90dim and cytokeratin negative. They appear to progress to larger CD90bright cytokeratin negative cells. As cytokeratin intensity increases from negative to dim to bright, the intensity of CD90 staining decreases. The vast majority of cytokeratin bright cells express no stem/progenitor markers. This apparent differentiation pattern is entirely absent in the examples of high grade non small cell lung cancer and malignant pleural effusions. Here small resting stem cell marker+ cells can express dim or bright cytokeratin and the a significant proportion of large stem cell marker+ cells (progenitor cells) are cytokeratin bright.
Figure 2. Differentiation patterns in normal lung, and lung cancer tumor and pleural effusions.
The stem/progenitor markers are plotted versus the epithelial differentiation marker cytokeratin. Left panels are density plots; right panels are color evented dot plots of the same data, where cyan dots represent cells of low light scatter (low morphologic complexity), and magenta dots represent cells of high light scatter (high morphologic complexity). Black dots represent cells that are CD90−, CD117− and CD133−. The dots were layered in the order of prevalence, with cyan on top, magenta in the middle, and black on the bottom.
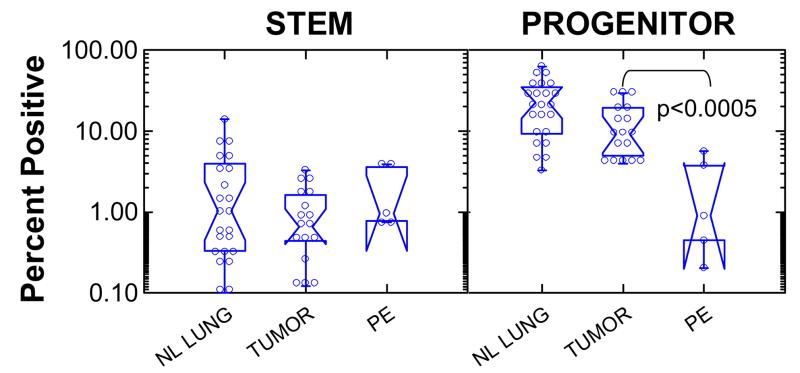
Figure 3 shows summary statistics of the frequency of stem cells and progenitor cells in normal lung, lung tumors, and pleural effusions. Frequencies are expressed as a percent of singlet cells with DNA content ≥ 2N. Stem cells were defined as cells with low light scatter (comparable to resting lymphocytes), expressing one or more stem/progenitor markers (CD90, CD119, CD133). Progenitor cells were stem/progenitor marker positive cells with high light scatter. Stem cell frequency was constant across normal lung and lung tumors (mean (lower, upper 95% confidence interval) = 0.92% (0.64, 1.34)). Progenitors comprised 18.9% (13.2, 27.1) of normal lung, 10.0% (6.97, 14.5) of tumor and 1.1% (0.20, 6.34) of pleural effusions. The progenitor content of pleural effusions was significantly lower than that of normal lung or lung tumor (p < 0.0005).
Figure 3. Frequency of stem and progenitor cells in normal lung, non-small cell lung tumors, and malignant pulmonary effusions.
Stem cells were defined as cells of low light scatter positive for one or more stem/progenitor cell markers (CD90, CD117, CD133). Progenitor cells were defined as stem/progenitor marker+ cells with high light scatter. Notched box plots display nonparametric (distribution-free) descriptive statistics. Individual data points are marked by open circles. The waist indicates the group median, the notch about the waist indicates the 95% confidence interval about the median. The hinges (upper and lower boundaries of the box) indicate interquartile distances. The whiskers (bars) give the ranges, exclusive of outliers.
MDR activity and in low and high grade malignancies
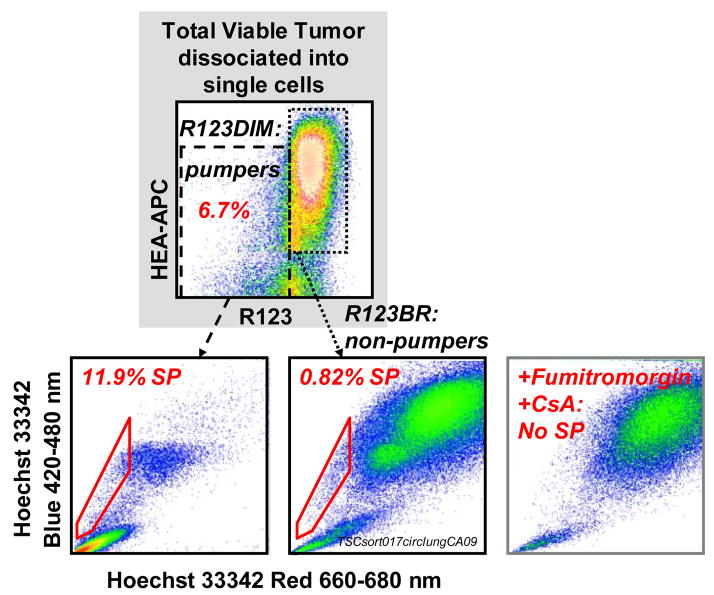
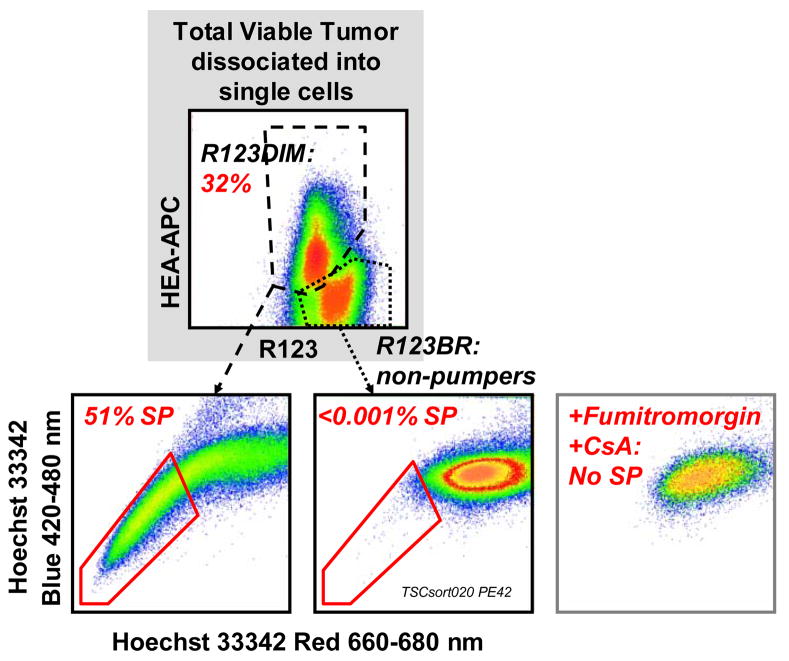
MDR activity has been used to identify and isolate stem cell enriched fractions from a variety of tissues. Figure 4 shows simultaneous measurement of the exclusion of two MDR substrates, Hoechst 33342 and rhodamine 123 on freshly isolated, therapy naïve non small cell lung cancer cells. Antibody stained suspended tumor cells were incubated simultaneously with Hoechst 33342 (8 microM) plus R123 (0.13 microM) for 90 minutes at 37°C. Propidium iodide (PI, 10 microg/mL) was added immediately before sample acquisition. All events were gated on PI excluding (live), non-hematopoietic CD45−) singlets (doublet discrimination based on forward light scatter pulse analysis). A total of 2.3 million cells were acquired.
Figure 4. ABCG2 and ABCB1 activity in freshly isolated therapy naïve non-small cell lung cancer.
Antibody stained suspended tumor cells were incubated simultaneously with Hoechst 33342 (8 microM) plus R123 (0.13 microM) for 90 minutes at 37°C. Propidium iodide (PI, 10 microg/mL) was added immediately before sample acquisition. All events were gated on PI excluding (live), non-hematopoietic CD45−) singlets (doublet discrimination based on forward light scatter pulse analysis). A total of 2.3 million cells were acquired.
This untreated newly diagnosed moderately well differentiated adenocarcinoma of the lung has a small proportion (6.7%) of cells which constitutively efflux R123. R123 is mainly transported by ABCB1. R123 dim (pumping) cells were heterogeneous for the expression of the epithelial differentiation marker HEA. They contain a significant subpopulation (11.9%), which co-transports the ABCG2 substrate Hoechst 33342. Cells which constitutively transport Hoechst 33342 have been termed the Side Population (SP). The great majority of R123bright cells was HEA+ and was almost devoid of SP cells. The R123dim and SP phenotypes were completely abrogated by the addition of the MDR inhibitors fumitremorgin plus cyclosporine, supporting the MDR transporter specificity of this functional assay.
Figure 5 shows an identical experiment performed using therapy naïve cells freshly isolated from a poorly differentiated, highly aggressive renal carcinoma metastatic to the lung. Unlike the previous example, The R123 efflux activity was present in a major population and exclusively confined to HEA+ cells. Among R123 effluxing cells, 51% co-transported Hoechst 33342. These data indicate a great expansion in the population with constitutive transporter activity, and breakdown in the orderly expression of stem cell-like transporter activity in differentiation marker negative cells.
Figure 5. ABCG2 and ABCB1 activity in freshly isolated therapy naïve malignant pleural effusion.
Antibody stained density gradient separated pleural effusion cells were incubated simultaneously with Hoechst 33342 and Rhodamine123 as described in Figure 4. Propidium iodide was used as a viability discrimination dye where all events were gated on PI excluding (live), non-hematopoietic CD45− singlets. A total of 3.9 and 2.6 million cells were acquired in 2 consecutive runs.
Discussion
Tumor differentiation grade and the cancer stem cell paradigm
Current descriptions of the cancer stem cell hypothesis make good intuitive sense with low grade, well differentiated tumors, where the majority of cells are non-tumorigenic deranged cells with a gene expression profile architectural remnants reflecting their tissue of origin. Within such tumors, careful microscopic inspection will reveal a rare proliferating progenitor cell population, discernable as mitotic figures. Flow cytometric analysis of dissociated tumor examining millions of cells reveals a still rarer population of stem cell marker positive resting cells, a proportion of which also have constitutive MDR activity and detoxifying enzyme activity (e.g. aldehyde dehydrogenase). This is shown in schematic in the left panel of Figure 6.
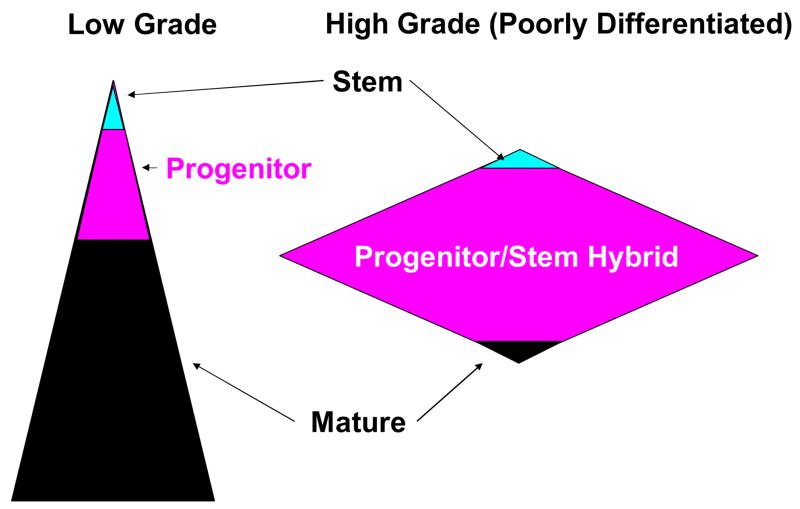
Figure 6. Differentiation patters in well and poorly differentiation malignancies.
Well differentiated malignancies resemble normal tissue and have distinct stem and progenitor compartments. Poorly differentiated malignancies retain a rare resting stem population, but lack a large population of cells bearing recognizable signs of maturation. Poorly differentiated cancers with a progenitor fraction that has acquired stem cell-like protective mechanisms (high MDR) will present as therapy resistant. Poorly differentiated cancers without a significant protected progenitor fraction proliferate aggressively but respond well to treatment. In both cases, resting cancer stem cells are spared by therapy.
At first blush, high grade, poorly differentiated tumors do not seem to fit the cancer stem cell paradigm. Proliferating cells are common, the tissue of origin is often difficult, and sometimes impossible to discern as histological cues and differentiation markers are often lacking. Here it is useful to remember that cancer is a disease of mutation and selection, and there is no predicting how much or how little of the normal differentiation pathway will be retained. For lack of better terminology, poorly differentiated tumors have a large population of differentiation deranged cells with properties of both stem and progenitor cells; these cells express both the proliferative capacity of the progenitor cell and the self-renewal of the stem cell (Figure 6 right panel). Whether they also retain the self-protection of the stem cell will determine whether they are intrinsically therapy resistant, or whether like some poorly differentiated hematologic malignancies, are exquisitely sensitive. It should be noted that neither of these scenarios in poorly differentiated disease preclude the retention of a vestigial population of tumor stem cells, with characteristic resting state and self-renewal and self-protective capacities. That these resting cells are present in poorly differentiated cancer is borne out by our experimental data (Figure 2) as well as clinical findings. In lung cancer, patients with poorly differentiated tumors tend to have better initial response to chemotherapy than those with well differentiated disease, but paradoxically, have shorter survival (19).
The most important contribution of the cancer stem cell hypothesis is the recognition that characteristics retained from normal tissue stem cells, or acquired as the result of multiple mutations, guarantee that a proportion of tumor cells will be intrinsically at least as therapy resistant as their normal tissue and hematopoietic counterparts. The selective pressure of therapeutic agents may further hone this resistance, but an intrinsically resistant population has been present in the tumor from its inception. Specifically, the characteristics that mediate intrinsic therapy resistance are: 1) The resting state, which renders cells resistant to agents dependent on DNA synthesis or high metabolic activity; 2) The lack of differentiation markers such as receptors (ER/PR, EGFR, VEGFR) and tissue specific proteins (tyrosine kinase) which constitute targets of therapy in more mature tumor cells; 3) Constitutive expression of MDR transporters; 4) Constitutive activity of detoxifying enzymes; 5) Resistance to apoptosis mediated by interaction of surface receptors and protective cells in the tumor environment (reviewed in 20).
Tumorigenicity versus tumor survival after therapy
It has long been recognized that only a fraction of tumor cells are tumorigenic. Successful cancer therapy requires eliminating all tumor populations from three distinct compartments: 1) Mature post mitotic tumor cells, which often interfere mechanically and biochemically with normal function; 2) Rapidly proliferating progenitor cells that are the source of bulky tumor; and 3) The rare resting, protected compartment of the tumor identified as cancer stem cells. It is important to distinguish between tumorigenic cells, which are not necessarily stem cells, and stem cells, which are not necessarily protected. The real culprit, and the population that routinely evades therapy is the tumorigenic, protected cell, and this is often a resting cell.
In support of this notion, we have previously shown that not all tumorigenic cells are stem cells (13), as defined by both expression of accepted stem cell markers and resting morphology. Neither is tumorigenicity enriched in tumor cells with constitutive MDR activity (21) nor expression (13). In contrast to tumor-derived cells, sorting for the constitutively MDR active “side population” greatly enriches normal stem cells from a variety of normal tissues (cited above 4–9).
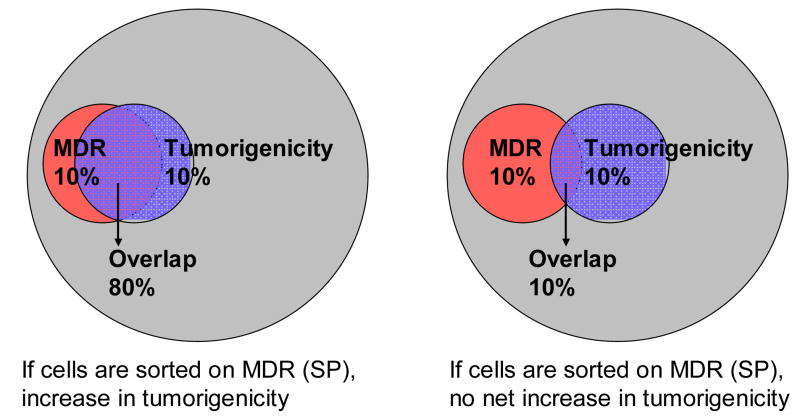
So why have experiments testing tumorigenicity of tumor derived side population cells failed to show enrichment of tumorigenic cells within this population (21)? Figure 7 shows graphically that the extent to which MDR activity and clonogenicity overlap will determine the effect of side population enrichment on tumorigenicity. In normal tissue stem cells not all stem cells are MDR protected (and therefore SP+), and not all SP+ cells are stem cells, but the overlap is considerable (left panel). This appears not to be the case in tumors, where both MDR activity and tumorigenicity appear to be rare events with limited overlap. Despite the uncoupling of MDR protection and tumorigenicity, there remains a rare MDR protected tumorigenic population which we propose to be the cell which evades apparently successful therapy.
Figure 7. Two scenarios concerning the relationship between tumorigenicity/clonogenicity and constitutive MDR expression.
On the left the frequency of MDR+ and tumorigenic cells are both 10%, but they overlap substantially. As in normal tissues, sorting MDR+ cells would yield a substantial enrichment in clonogenic cells. On the right MDR+ cells and tumorigenic cells also constitute 10% of the tumor population, but they only overlap by 10%. Sorting MDR+ cells in this case would result in no enrichment of tumorigenic cells. Importantly, in both cases a fraction of tumorigenic cells are protected by constitutive MDR activity and therefore inherently therapy resistant.
Therapeutic Index Revisited
MDR activity has been proposed as a major mechanism responsible for sparing normal tissue stem cells from the effects of chemotherapy, which often kills both the bulk of tumor cells as well as actively metabolizing normal cells in the bone marrow, skin and gut. Therapeutic agents, in fact, are dosed to prevent irreversible damage to normal tissue. This translates to preventing irreversible damage to tissue stem cells. A dramatic example can be found in chemotherapy-induced alopecia, which results from damage to the progenitor cells of the hair follicle. These cells are sensitive to antimitotic and antimetabolic agents because of their rapid turnover. However, alopecia is reversed on cessation of therapy, precisely because the four distinct cell types which comprise the follicle, as well as skin epithelial cells themselves, are all derived from a common progenitor, which itself is derived from an MDR-protected epithelial stem cell.
Given the paradigm shown in the left hand panel of Figure 6, the closer resting cancer stem cells resemble their normal counterparts, the less likely MDR substrate drugs, or non-substrate agents that target proliferating cells, receptors or enzymes in metabolically active cells, will be effective. Herein lies the conundrum of therapeutic index. The efficacy of an anti-neoplastic agent or regimen is judged by its ability to shrink tumors without irreversible damage to normal tissue. However, successful treatment is often defined as the ability to achieve a durable remission. The very fact that cancers can and do relapse after apparently successful therapy indicates the survival of an occult treatment-resistant tumorigenic population. Therapeutic index must therefore be redefined as the differential toxicity to cancer stem cells, relative to tissue stem cells. This is a much more difficult problem because it requires agents which can exploit biological differences between cancer and normal stem cells, rather than the numerous disparities between metabolically active tumor cells and resting normal stem cells.
Acknowledgments
This work was supported by grants BC032981 and BC044784 from the Department of Defense, the Hillman Foundation and the Glimmer of Hope Foundation. Vera Donnenberg is a CDMRP Era of Hope Scholar.
The authors would like to thank Ms. Darlene Monlish and Ms. Melanie Pfeifer and Mr. E. Michael Meyer for their expert technical assistance. We would also like to thank Mr. Peter Nobes and Mr. David Roberts of Applied Cytometry Systems for the opportunity to collaborate on the development of software specifically designed for multiparameter rare event analysis on large datafiles.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Leslie EM, Deeley RG, Cole SP. Multidrug resistance proteins: role of P-glycoprotein, MRP1, MRP2, and BCRP (ABCG2) in tissue defense. Toxicology & Applied Pharmacology. 2005;204(3):216–37. doi: 10.1016/j.taap.2004.10.012. [DOI] [PubMed] [Google Scholar]
- 2.Biedler JL, Riehm H. Cellular resistance to actinomycin D in Chinese hamster cells in vitro: cross-resistance, radioautographic, and cytogenetic studies. Cancer Research. 1970;30(4):1174–84. [PubMed] [Google Scholar]
- 3.Ling V, Thompson LH. Reduced permeability in CHO cells as a mechanism of resistance to colchicine. Journal of Cellular Physiology. 1974;83(1):103–16. doi: 10.1002/jcp.1040830114. [DOI] [PubMed] [Google Scholar]
- 4.Udomsakdi C, Eaves CJ, Sutherland HJ, Lansdorp PM. Separation of functionally distinct subpopulations of primitive human hematopoietic cells using rhodamine-123. Exp Hematol. 1991;(5):338–42. [PubMed] [Google Scholar]
- 5.Goodell MA, Brose K, Paradis G, Conner AS, Mulligan RC. Isolation and functional properties of murine hematopoietic stem cells that are replicating in vivo. J Exp Med. 1996;183(4):1797–806. doi: 10.1084/jem.183.4.1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Giangreco A, Shen H, Reynolds SD, Stripp BR. Molecular phenotype of airway side population cells. Am J Physiol Lung Cell Mol Physiol. 2004;286(4):L624–30. doi: 10.1152/ajplung.00149.2003. [DOI] [PubMed] [Google Scholar]
- 7.Chen J, Hersmus N, Van Duppen V, Caesens P, Denef C, Vankelecom H. The adult pituitary contains a cell population displaying stem/progenitor cell and early embryonic characteristics. Endocrinology. 2005;146(9):3985–98. doi: 10.1210/en.2005-0185. [DOI] [PubMed] [Google Scholar]
- 8.He DN, Qin H, Liao L, Li N, Zhu WM, Yu BJ, Wu X, Zhao RC, Li JS. Small intestinal organoid-derived SP cells contribute to repair of irradiation-induced skin injury. Stem Cells & Development. 2005;14(3):285–91. doi: 10.1089/scd.2005.14.285. [DOI] [PubMed] [Google Scholar]
- 9.Riou L, Bastos H, Lassalle B, Coureuil M, Testart J, Boussin FD, Allemand I, Fouchet P. The telomerase activity of adult mouse testis resides in the spermatogonial alpha6-integrin-positive side population enriched in germinal stem cells. Endocrinology. 2005;146(9):3926–32. doi: 10.1210/en.2005-0502. [DOI] [PubMed] [Google Scholar]
- 10.Donnenberg VS, Donnenberg AD. Multiple Drug Resistance in Cancer Revisited: The Cancer Stem Cell Hypothesis. J Clin Pharmacol. 2005;45:872 – 877. doi: 10.1177/0091270005276905. [DOI] [PubMed] [Google Scholar]
- 11.Chaudhary PM, Roninson IB. Expression and activity of P-glycoprotein, a multidrug efflux pump, in human hematopoietic stem cells. Cell. 1991;12;66(1):85–94. doi: 10.1016/0092-8674(91)90141-k. [DOI] [PubMed] [Google Scholar]
- 12.Bertoncello I, Williams B. Hematopoietic Stem Cell Characterization by Hoechst 33342 and Rhodamine 123 Staining. In: Hawley TS, Hawley RG, editors. Methods in Molecular Biology: Flow Cytometry Protocols. 2. Humana Press Inc; Totowa, NJ: 2004. pp. 181–200. [DOI] [PubMed] [Google Scholar]
- 13.Donnenberg VS, Luketich JD, Landreneau RJ, DeLoia JA, Basse P, Donnenberg AD. Ernst Schering Res Foundation Workshop. 2006. Tumorigenic Epithelial Stem Cells and Their Normal Counterparts. In Press. [DOI] [PubMed] [Google Scholar]
- 14.Elder EM, Whiteside TL. Processing of Tumors for Vaccine and/or Tumor Infiltrating Lymphocytes. In: Rose NR, Conway de Macario E, Fahey JL, Friedman H, Penn GM, editors. Manual of Clinical Laboratory Immunology. 4. American Society for Microbiology; 1992. pp. 817–819. [Google Scholar]
- 15.Donnenberg VS, Donnenberg AD. Identification, rare-event detection and analysis of dendritic cell subsets in broncho-alveolar lavage fluid and peripheral blood by flow cytometry. Frontiers in Bioscience. 2003;8:1175–1180. doi: 10.2741/1185. [DOI] [PubMed] [Google Scholar]
- 16.Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proceedings of the National Academy of Sciences of the United States of America. 2003;100(7):3983–8. doi: 10.1073/pnas.0530291100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li C, Heidt DG, Dalerba P, Burant CF, Zhang L, Adsay V, Wicha M, Clarke MF, Simeone DM. Identification of Pancreatic Cancer Stem Cells. Cancer Res. 2007;67(3):1030–1037. doi: 10.1158/0008-5472.CAN-06-2030. [DOI] [PubMed] [Google Scholar]
- 18.Singh SK, Clarke ID, Terasaki M, Bonn VE, Hawkins C, Squire J, Dirks PB. Identification of a Cancer Stem Cell in Human Brain Tumors. Cancer Research. 2003;63:5821–5828. [PubMed] [Google Scholar]
- 19.Saijo N, Niitani H, Tominaga K, Eguchi K, Koketsu H, Fujino T, Ishikawa S. Comparison of survival in nonresected well differentiated and poorly differentiated adenocarcinoma of the lung. Journal of Cancer Research and Clinical Oncology. 1980;97(1):71–79. doi: 10.1007/BF00411280. [DOI] [PubMed] [Google Scholar]
- 20.Moore KA, Lemischka IR. Stem Cells and Their Niches. Science. 2006;311:1880–1885. doi: 10.1126/science.1110542. [DOI] [PubMed] [Google Scholar]
- 21.Szotek PP, Pieretti-Vanmarcke R, Masiakos PT, Dinulescu DM, Connolly D, Foster R, Dombkowski D, Preffer F, MacLaughlin DT, Donahoe PK. Ovarian cancer side population defines cells with stem cell-like characteristics and Mullerian Inhibiting Substance responsiveness. PNAS. 2006;103:11154–11159. doi: 10.1073/pnas.0603672103. [DOI] [PMC free article] [PubMed] [Google Scholar]