Figure 1. Protein Trafficking from the ER.
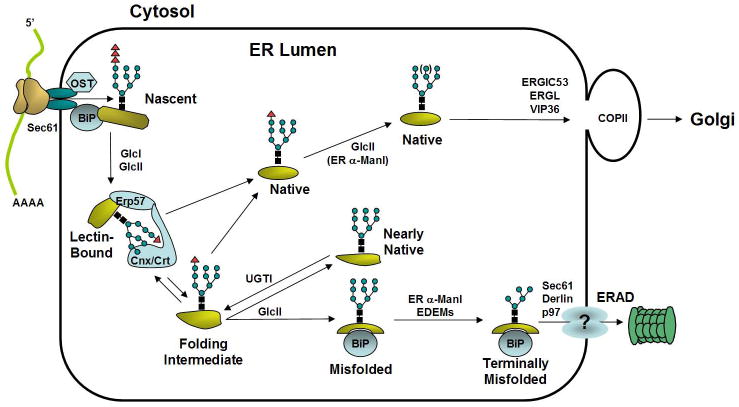
Upon translocation of polypeptides through the Sec61 proteinaceous channel, asparagine residues are frequently modified by covalent addition of a preassembled oligosaccharide core (N-acetylglucosamine2-mannose9-glucose3). This reaction is catalyzed by the oligosaccharyltransferase (OST), a multisubunit complex associated with translocon. To facilitate unidirectional transport through the translocon, nascent polypeptide chains in the ER lumen interact with BiP, a molecular chaperone that binds to exposed hydrophobic residues. Subsequently, rapid deglucosylation of the two outermost glucose residues on the oligosaccharide core structures, mediated by glucosidase I and II (GlcI and GlcII), prepares glycoproteins for association with the ER lectins calnexin and calreticulin. The calnexin/calreticulin-associated oxidoreductase ERp57 facilitates protein folding by catalyzing formation of intra- and inter- molecular disulfide bonds, a rate-limiting step in the protein folding process. Release from calnexin/calreticulin followed by glucosidase II cleavage of the innermost glucose residue prevents further interaction with calnexin and calreticulin. At this point, natively folded polypeptides transit the ER to the Golgi compartment, in a process possibly assisted by mannose-binding lectins, such as ERGIC-53, VIPL, ERGL. As an essential component of protein-folding quality control, non-native polypeptides are tagged for reassociation with calnexin/calreticulin by the UDP-glucose:glycoprotein glucosyltransferase (UGT1) to facilitate their ER retention and prevent anterograde transport. Polypeptides that are folding incompetent are targeted for degradation by retrotranslocation, possibly mediated by EDEM and Derlins, into the cytosol and delivery to the 26S proteosome. Triangles represent glucose residues, squares represent N-acetylglucosamine residues, and circles represent mannose residues.
