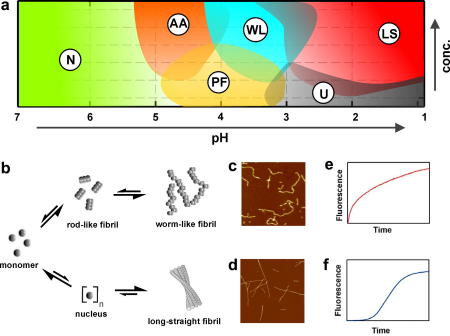
Fig. 4.
Pathway complexity of β2m amyloid fibril formation. (a) Schematic state diagram representing the different thermodynamic ground states observed upon incubation of β2m under different conditions. While the native protein (N) remains monomeric even at high protein concentration, acidification results in the protein unfolding to form partially folded (PF) or more highly unfolded forms (U). Above a critical concentration these species self-associate, forming amyloid fibrils with distinct morphological properties. Close to the protein’s pI, amorphous aggregates (AA) are formed, while worm-like fibrils (WL) and classic long-straight fibrils (LS) are formed at lower pH values. (b) Proposed model for competing pathways that lead to the formation of worm-like fibrils or long-straight amyloid fibrils. (c,d) AFM images of worm-like (c) and long-straight fibrils (d). All images are 1 μm2. (e,f) Worm-like fibrils form with nucleation-independent kinetics (e), whilst the formation of long-straight fibrils is nucleation-dependent and shows a clear lag-phase (f). Figure adapted from [126] with permission.