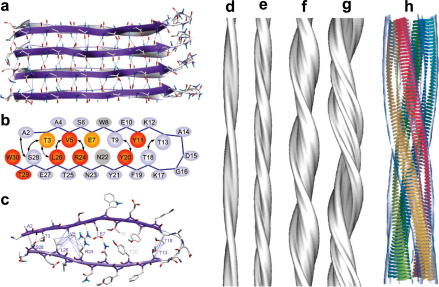
Fig. 5.
General structural motifs of amyloid-like fibrils. (a–c) Structural model for protofilaments formed in vitro by a WW domain. (a) Perpendicular view to the fibril axis, indicating the continuous β-sheet hydrogen bond structure (dotted lines). (b) Cartoon representation of the non-native β-strand-loop-β-strand motif adopted in the amyloid structure. Structural restraints from ssNMR measurements are indicated by arrows and side chains eliminating (red) or reducing (orange) amyloid fibril formation when mutated to alanine are highlighted. (c) Atomistic representation of the tight packing between strands (viewed along the fibril axis as in (b)). (d–g) Surface representation of 3D maps obtained using cryoEM for insulin fibril structures. The fibrils contain either two (d), four (e) or six (f and g) protofilaments. (h) Proposed β-strand model for insulin fibrils containing four protofilaments. Figure adapted from references [119] and [87] with permission.