Figure 7.
Model for the Structure and Priming Stroke of Dynein
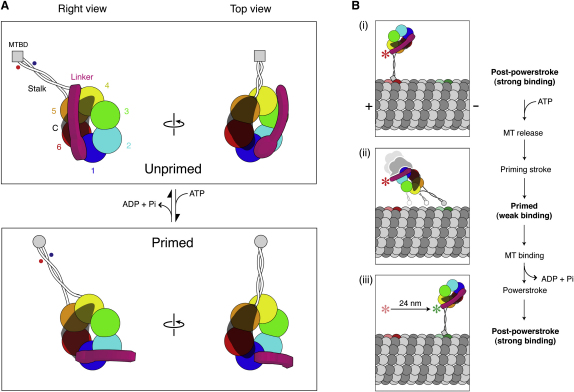
(A) Six AAA+ modules (numbered) form a hexameric ring. The C sequence (translucent black) is represented speculatively as an elongated structure interacting with (one or another face of) AAA6, AAA5, and AAA4. The N sequence (magenta) contains the linker which runs from AAA1 across the head to AAA4 (yellow) in the unprimed conformation and switches to a position close to AAA2 in the primed conformation, thereby moving the N terminus of the motor by ∼17 nm (in right view in a plane parallel to the page). The N sequence may also contain nonlinker structures, such as near the junction with AAA1 (magenta ellipse). Tilting of the stalk, shown here to occur entirely in the plane of the page in right view (see Figure S3), displaces the MTBD by ∼5 nm and could shift the registration of the two α helices of the coiled coil (indicated by red and blue spots). Stalk tilting perpendicular to the page is not seen in top-view data (Figure 5C), probably because the stalk flattens down onto the EM grid in this orientation.
(B) Model to illustrate how the linker swing and stalk tilt could produce one of dynein's larger displacements along an MT (see Discussion for further details). The attachment geometry proposed here (i and iii) is compatible with Mizuno et al. (2007) and uses the right view, which ensures that movement of the linker N terminus occurs in a plane parallel to the MT axis. With the linker N terminus initially restrained (red asterisk), perhaps by attachment to the second head as observed in dimeric dynein-f (Kotani et al., 2007), ATP binding (ii) causes MT detachment and the priming stroke which displaces the MTBD along the MT, here by 24 nm. Subsequent reattachment and powerstroke (iii) displaces the linker N terminus by 24 nm (green asterisk). α-β tubulin dimers are shown (to scale) as pairs of dark and light gray spheres with red and green dimers showing initial and final binding sites, respectively.