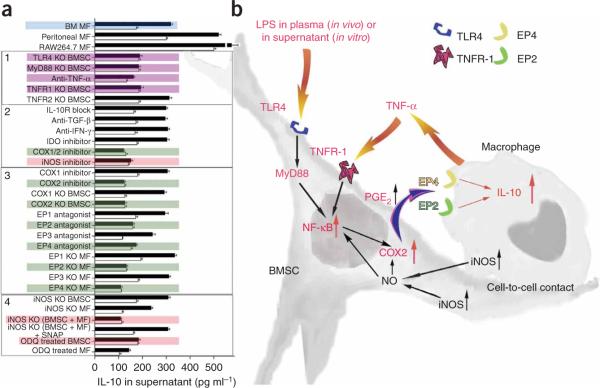
Figure 6.
Summary of studies of the molecular pathways involved in the interaction between BMSC and macrophages. (a) IL-10 concentration changes in supernatants of cocultures in a variety of treatment conditions after LPS stimulation. Colored graphs (except for the blue color that labels the bone marrow macrophages as the source of all consecutive experiments) show treatments that eliminate the effect of BMSCs on macrophages. Black graphs show IL-10 levels after LPS stimulation, whereas open graphs show the control (nonstimulated) values. The experiments where the conditions eliminated the effect are colored. Purple color shows the effect of septic environment, green color shows agents and cellular compartments related to the PGE2 pathway and pink color shows agents related to the nitric oxide pathway. Three separate kinds of macrophages (bone marrow macrophages; peritoneal macrophages and the RAW264.7 cell line) were examined initially. Because they behaved identically in the assay, we used bone marrow derived macrophages (BM MF) for the rest of the experiments. In the box labeled 1, the effect of septic environment on the BMSCs is studied in BMSCs from TLR4-, MyD88-, TNFR1- and TNFR2- deficient mice, or antibody to TNF-α was used to neutralize the effect of TNF. The box labeled with 2 shows the cytokines and agents that have been implicated in the literature in immunomodulation of T cells by BMSCs, including COX1/2 and iNOS inhibitors. The box labeled 3 shows studies of the COX2 pathway, including the prostaglandin receptors EP1–EP4. Finally, in the box labeled with 4, we show studies related to nitric oxide. (b) A summary of our current hypothesis about the mechanisms that underlie the interactions between BMSCs and macrophages in the CLP sepsis model. Bacterial toxins (for example, LPS) and circulating TNF-α act on the TLR4 and TNFR-1 of the BMSCs, respectively. This results in the translocation of NF-κB into the nucleus. This activation process seems to be nitric oxide dependent. Activated NF-κB induces the production of COX2, resulting in increased production and release of PGE2. PGE2 binds to EP2 and EP4 receptors on the macrophage, increasing its IL-10 secretion and reducing inflammation.