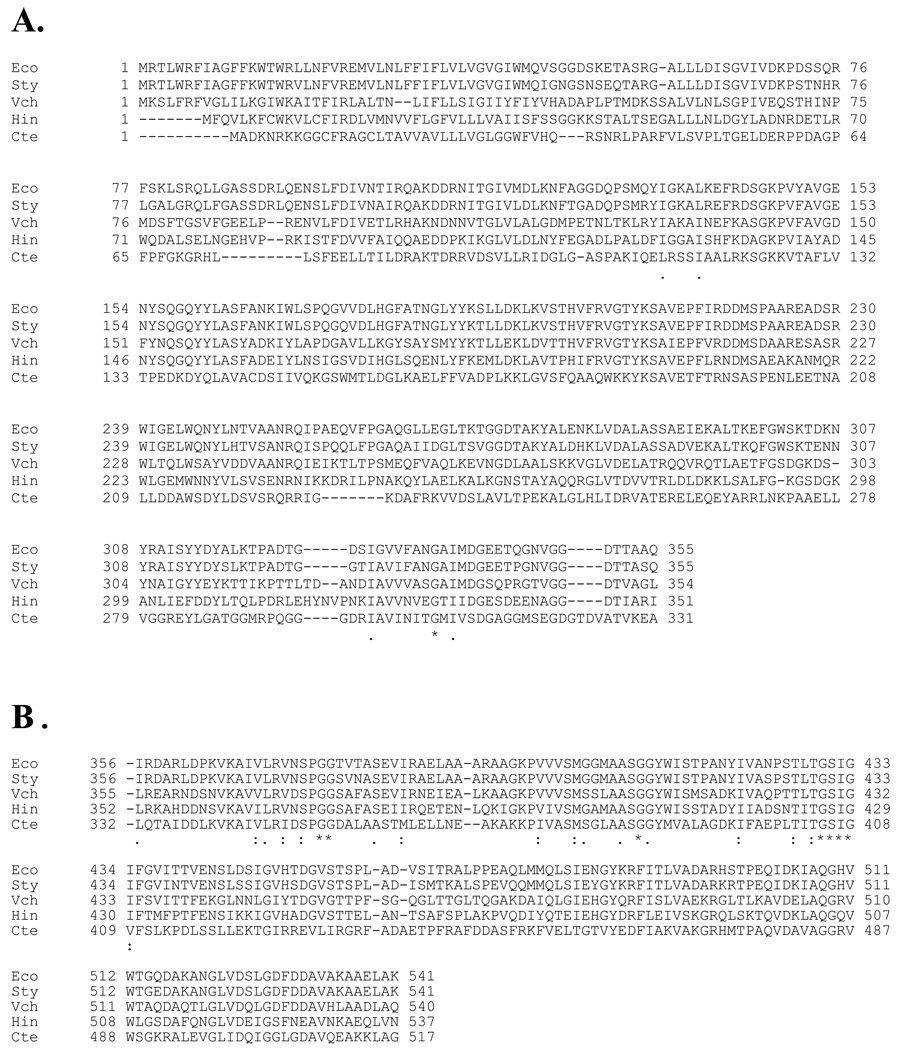
FIGURE 1. Multiple alignment of the amino acid sequences of SppA S49.001 members.
A. The amino-terminal domain within the S49.001 family. B. The carboxyl-terminal protease domain within the S49.001 family. Shown are the sequences for Eco, Escherichia coli; Sty, Salmonella typhimurium; Vch, Vibrio cholerae; Hin, Haemophilus influenzae; Cte, Chlorobium tepidum. Shown below the sequences are the consensus motifs for the entire S49.001 group. “*” denotes that the amino acids in the S49.001 are identical in all sequences in the alignment. “:” means that conserved substitutions in the S49.001 group have been observed, according to the nature of the amino acid (hydrophobic, hydrophilic, acidic or basic).“.” means that semi-conserved substitutions in the S49.001 group are found. The shaded residues in the carboxyl-terminal domain are invariant in the S49 family. The shaded residue in the amino-terminal domain is absolutely conserved when the amino-terminal domain is present.