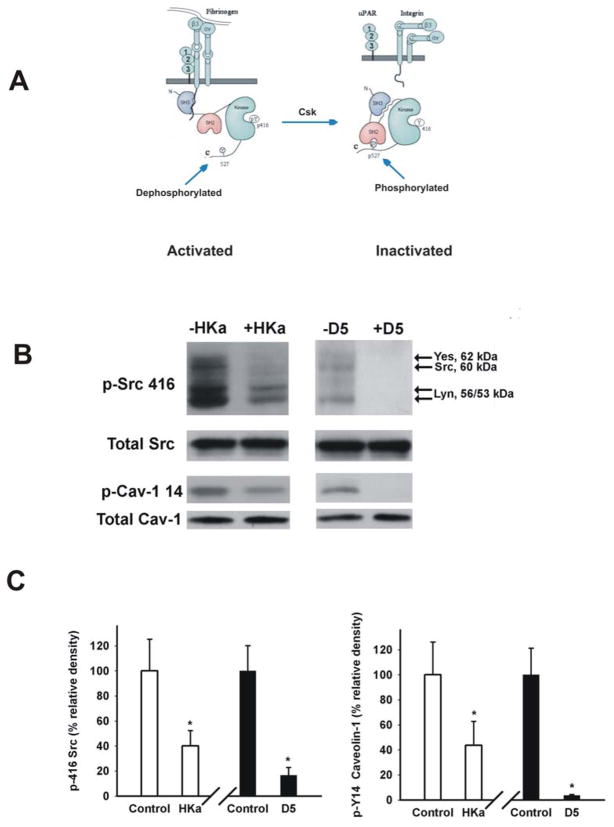
Figure 4. HKa and D5 inhibited Tyr 416 phosphorylation of Src family kinases.
A, fibrinogen binds to the integrin αvβ3 or α5β1, resulting the conformational change of integrins. The outside-in signal of integrin αvβ3 induces the association of SH3 domain of Src with the intracellular tail of integrin β3. This association results from Src activation by dephosphorylation of Tyr 527 and auto-phosphorylation of Tyr416. In contrast, Csk inactivates Src by phosphorylating Tyr 527. B, HUVECs were cultured in 3D collagen-fibrinogen gel matrices for 22 hours at 37°C in the presence of angiogenic stimulators plus 300 nM HKa or D5. The gel containing cells was washed once with ice-cold Dulbecco’s PBS and then the cells were lyzed by adding extraction buffer. The gel was removed by centrifugation at 5000×g for 5min at 4°C. The protein concentration was measured by Coomassie Blue Plus (PIERCE). Proteins were separated on SDS PAGE and membranes were probed with an antibody to Src phospho-tyrosine 416 as well as an antibody to caveolin-1 phospho-tyrosine 14. Total Src and caveolin-1 showed equal loading. C, Src phospho-tyrosine 416 and caveolin-1 phospho-tyrosine 14 were quantified by densitometry. The data were collected from three independent experiments, represented as mean ± SEM (*p<0.05 compared to control).