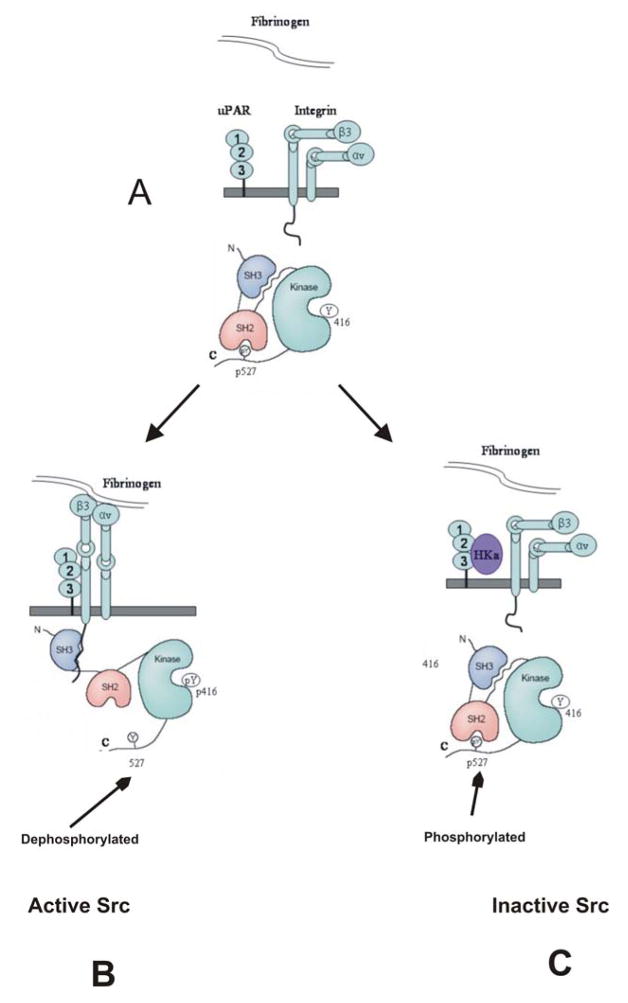
Figure 8. Illustration of Src activity modulated by HKa in response to outside-in signaling of αvβ3 integrin.
A, Src remains an inactive form where SH2 domain is engaged with phosphorylated Tyr 527, SH3 domain is engaged with the SH2-kinase-linker and Tyr416 (SH1) is unphosphorylated. B, the binding of fibrinogen to the αvβ3 integrin on the cell membrane results the conformational change of integrin αvβ3 and initiates the outside-in signaling, which induces the association of the intracellular tail of the β3 integrin with SH3 domain of Src. This association facilitates de-phosphorylation of pTyr 527 and auto-phosphorylation of Tyr 416 in Src kinase, which results in Src activation. The clustering of uPAR to αvβ3 integrin in response to integrin activation modulates the bidirectional signaling of αvβ3 integrin. C, addition of HKa decreases Src kinase activity by targeting uPAR.