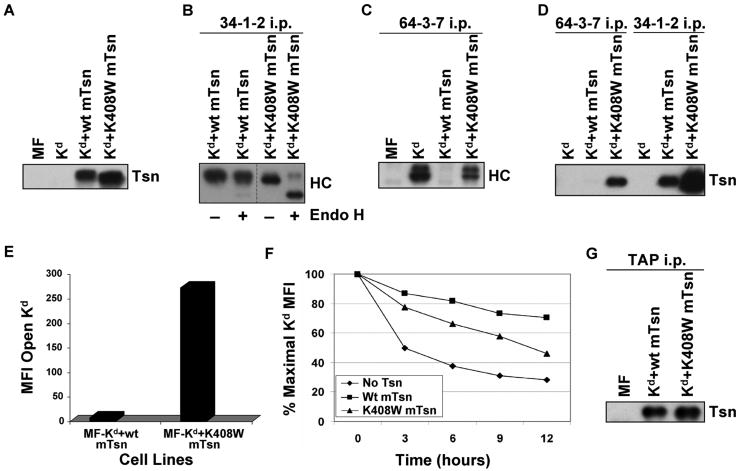
Figure 6.
(A) Tapasin K408W was detected in similar or slightly greater amount than wild type tapasin in the transfected cells. Samples of cell lysates were electrophoresed on a 10% acrylamide Tris-glycine gel, and the proteins were transferred to a blotting membrane and probed with an antibody for mouse tapasin (Tsn). (B) A majority of the Kd molecules folded in the presence of mouse tapasin K408W were Endo H sensitive. Folded Kd molecules were immunoprecipitated with 34-1-2, electrophoresed on 4→20% acrylamide Tris-glycine gel, blotted, and probed with 64-3-7 (HC). Samples were treated (+) or untreated (-) with Endo H as indicated. (C) Mouse tapasin K408W was found to increase the amount of Kd in the open conformation. Immunoprecipitations were performed with antibody 64-3-7 on lysates of the indicated cell types. The immunoprecipitated proteins were electrophoresed on a 4→20% acrylamide Tris-glycine gel, transferred, and probed on a blot with 64-3-7 (HC). (D) Kd was more strongly associated with tapasin K408W than with wild type tapasin, and both wild type tapasin and tapasin K408W were predominantly associated with the folded, and not the open, form of Kd. Immunoprecipitations were performed with mAb 64-3-7 and 34-1-2 on lysates of the indicated cell types. The immunoprecipitated proteins were electrophoresed on a 10% acrylamide Tris-glycine gel, transferred to a membrane, and probed with anti-tapasin antibody (Tsn). (E) The amount of open Kd at the cell surface was increased by expression of tapasin K408W compared to wild type tapasin. Cells were incubated with secondary antibody only, or with an antibody against the open form of the Kd molecule (64-3-7). Background staining with PE-conjugated secondary antibody only was <5.0 for both MF-Kd+wt mTsn and MF-Kd+K408W mTsn (not shown). Values on the y axis are relative mean fluorescence intensity (MFI) units. Similar results were obtained in a separate flow cytometry experiment. (F) Kd molecules assembled in cells expressing mouse tapasin K408W were less stable than those assembled in the presence of wild type mouse tapasin. Cells were treated with 2 μg/ml Bfa in complete medium for 0, 3, 6, 9, or 12 hours, then stained with 34-1-2 and analyzed by flow cytometry. (G) The K408W mutation in mouse tapasin does not abrogate the association of tapasin with TAP. Immunoprecipitations were performed with an anti-TAP antiserum on lysates of the cell lines indicated. The immunoprecipitated proteins were electrophoresed on a 10% acrylamide Tris-glycine gel, and probed on a Western blot with an anti-mouse tapasin antibody.