Fig. 1.
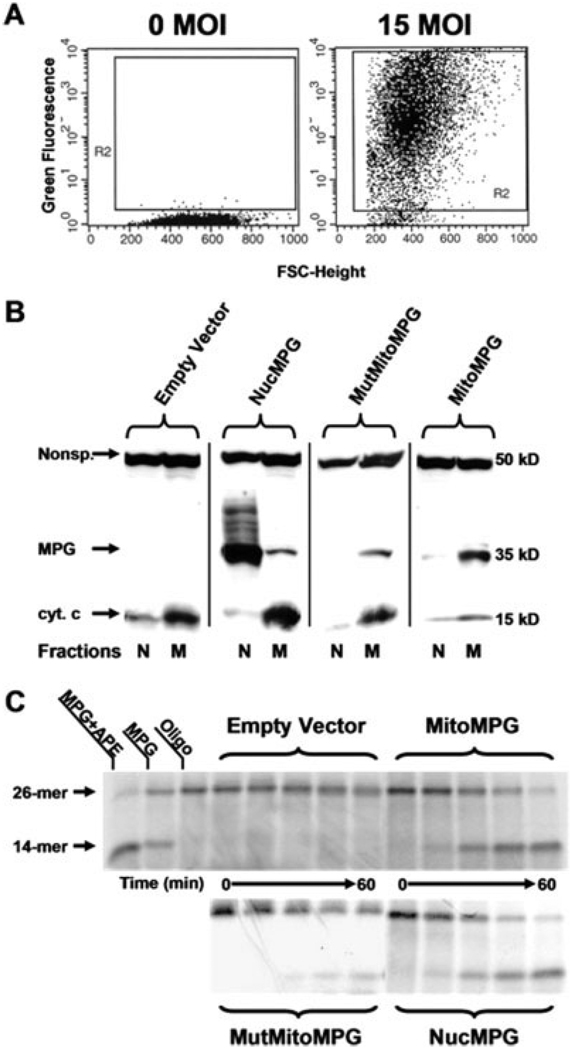
Efficient transduction of the MPG gene in primary astrocyte cultures using an adenoviral expression system. A: FACS analysis of EGFP expression in samples of 10,000 cells taken from nontransduced (0 MOI) and transduced (15 MOI) cultures of primary astrocytes. B: Western blot analysis confirms appropriate subcellular localization of human MPG to either the nucleus (N) or mitochondria (M) in transduced rat astrocyte cultures. Anticytochrome c antibody was used to show that the cell fractions were either mitochondrial or nuclear protein enriched. Additionally, a nonspecific band that immunoreacts with the cyt. c antibody was included to show comparable protein loading between fractions. The human MPG antibody used in this study does not immunoreact with rat MPG. C: The oligonucleotide cleavage assays were performed using a 26-mer oligonucleotide containing a 1,N6-ethenoadenine adduct. The radiolabeled oligo was incubated at 37°C with either nuclear lysates (NucMPG) or mitochondrial lysates (empty vector, MitoMPG and MutMitoMPG) for 0, 5, 20, 40, and 60 min. The reaction products were resolved on a 15% TBE-urea gel and autoradiography was performed. One unit of purified recombinant mouse Aag (MPG) was used as a positive control as well as 1 unit of purified human APE.