Abstract
BACKGROUND:
Diabetes currently affects more than 7% of the Canadian population, and heart failure is a well-documented complication of diabetes. The medical management of heart failure is often limited by disease progression, and cardiac transplantation is a key therapeutic option in end-stage disease. However, both American and Canadian guidelines continue to list diabetes as a relative contraindication to cardiac transplantation.
OBJECTIVE:
To determine the effect of preoperative diabetes on morbidity and mortality in patients undergoing cardiac transplantation.
METHODS:
A retrospective analysis of 136 adult patients undergoing cardiac transplantation at the London Health Sciences Centre (London, Ontario) between February 1995 and November 2003 was performed. Preoperatively, 14% of patients were diabetic. Unpaired Student’s t tests and χ2 tests were used to compare outcomes between diabetic and nondiabetic cardiac transplant recipients.
RESULTS:
Diabetic and nondiabetic cardiac transplant recipients were similar in age, sex, body mass index and ischemic time. Preoperatively, diabetic recipients had a higher mean serum glucose and an increased incidence of ischemic cardiomyopathy. At three years postcardiac transplantation, diabetic recipients were found to have increased rates of transplant coronary artery disease, as well as decreased cardiac function. However, diabetic and nondiabetic patients showed no differences in rates of clinically significant infection or rejection in the first three postoperative months. Furthermore, survival rates were similar between these two groups.
CONCLUSION:
Diabetes is not a contraindication to cardiac transplantation, but increased vigilance is warranted in this population to minimize postoperative morbidity.
Keywords: Diabetes mellitus, Morbidity, Mortality, Transplantation
Abstract
HISTORIQUE :
Le diabète touche plus de 7 % de la population canadienne, et l’insuffisance cardiaque est une complication bien documentée du diabète. La prise en charge de l’insuffisance cardiaque est souvent limitée par l’évolution de la maladie, et la greffe cardiaque est une option thérapeutique importante de la maladie en phase finale. Cependant, les lignes directrices américaines et canadiennes continuent de répertorier le diabète parmi les contre-indications relatives de la greffe cardiaque.
OBJECTIF :
Déterminer les effets du diabète préopératoire sur la morbidité et la mortalité des patients qui subissent une greffe cardiaque.
MÉTHODOLOGIE :
Les auteurs ont procédé à une analyse rétrospective de 136 patients adultes qui ont subi une greffe cardiaque au London Health Sciences Centre de London, en Ontario, entre février 1995 et novembre 2003. Avant l’opération, 14 % des patients étaient diabétiques. Les auteurs ont utilisé les tests t et du chi carré non appariés de Student pour comparer les issues entre les greffés cardiaques diabétiques et non diabétiques.
RÉSULTATS :
Les greffés cardiaques diabétiques et non diabétiques étaient d’âge, de sexe, d’indice de masse corporelle et de période d’ischémie similaires. Avant l’opération, les personnes diabétiques présentaient un glucose sérique moyen plus élevé et une plus forte incidence de myocardiopathie ischémique. Trois ans après la greffe cardiaque, les auteurs ont constaté que les patients diabétiques présentaient un taux plus élevé de coronaropathie de la greffe, ainsi qu’une diminution de la fonction cardiaque. Cependant, il n’y avait pas de différence clinique significative d’infection ou de rejet pendant les trois premiers mois postopératoires chez les patients diabétiques et non diabétiques. De plus, les taux de survie étaient similaires dans les deux groupes.
CONCLUSION :
Le diabète n’est pas une contre-indication à la greffe cardiaque, mais il faut se montrer plus vigilant auprès de cette population afin de réduire la morbidité postopératoire au minimum.
Diabetes mellitus is reaching epidemic proportions worldwide and currently affects more than 7% of the Canadian population (1). Heart failure is a well-documented complication of diabetes, occurring twice as often in diabetic men and five times as often in diabetic women compared with age-matched controls (2). The medical management of heart failure is often limited by disease progression, and cardiac transplantation is a key therapeutic option in end-stage disease (3). However, both American (4) and Canadian (5) guidelines continue to list diabetes mellitus as a relative contraindication to cardiac transplantation.
Historically, there has been concern that diabetes mellitus in cardiac transplant recipients increases the risk of infection, rejection, transplant coronary artery disease (TCAD), renal failure and mortality. However, the majority of previous studies, despite large subject numbers, have failed to document significant differences in morbidity between diabetic and nondiabetic cardiac transplant recipients (6–11). Additionally, only a few studies have demonstrated increased mortality in diabetic recipients (12–15).
In the present study, we reviewed our recent experience with cardiac transplantation to determine the relationship between pre-operative diabetes and intermediate-term morbidity and mortality. In addition to previously studied outcomes, including survival, renal function, TCAD, infection and rejection, we also evaluated cardiac function.
METHODS
Between February 1995 and November 2003, 143 adults underwent cardiac transplantation at the London Health Sciences Centre, University Campus (London, Ontario). Of these 143 patients, 136 had complete serial follow-up performed at the centre, and thus were included in the study. Patients were classified as either diabetic or non-diabetic. Diabetes was defined as requiring insulin, oral hypoglycemics or a diabetic diet before transplantation. Preoperatively, 14% of patients were diabetic. All patients were screened extensively for end organ damage, including microvascular and macrovascular complications. Each patient was reviewed and considered on an individual basis.
A chart review was performed to analyze survival, renal function, cardiac function, TCAD, and clinically significant rejection and infection. Cardiac function at one and three years following transplantation was determined by left ventricular ejection fraction (LVEF), measured by nuclear medicine wall motion studies. TCAD was defined as any luminal irregularity or more severe lesion seen on a coronary angiogram at one and three years post-transplantation. Both nuclear medicine wall motion studies and coronary angiography were performed routinely at one- and three-year follow-up examinations. Clinically significant rejection was defined as any rejection episode within the first three months of transplantation requiring an alteration in the immunosuppressive regimen, while clinically significant infection was defined as any infection requiring antibiotics and/or hospitalization within the first three months. Infection and rejection were monitored for the first three postoperative months only; based on previous experience, this is the period with highest risk for these complications.
Diabetic and nondiabetic transplant recipients were compared using unpaired Student’s t tests for means and χ2 tests for proportions. Kaplan-Meier analysis was used to determine actuarial survival. P≤0.05 was considered to be statistically significant.
RESULTS
Table 1 lists the preoperative clinical characteristics of diabetic and nondiabetic cardiac transplant recipients. There were no significant differences in age, sex or body mass index. All preoperative blood work, including cholesterol, was similar between the two groups, except for random serum glucose, which was significantly elevated in diabetic patients (13.0±7.0 mmol/L versus 6.2±1.1 mmol/L; P<0.001). Compared with nondiabetic recipients, diabetic patients had an increased incidence of ischemic cardiomyopathy as the etiology of their heart failure (53% versus 27%; P=0.02). Furthermore, there was no significant difference between diabetic and nondiabetic recipients with respect to donor heart ischemic time (229±80 min versus 221±91 min, respectively; P=0.73).
TABLE 1.
Preoperative clinical characteristics of diabetic and nondiabetic cardiac transplant recipients
| Characteristics | Diabetic patients (n=19) | Nondiabetic patients (n=117) | P |
|---|---|---|---|
| Age, years* | 54±7 | 50±12 | 0.11 |
| Male sex, % | 79 | 82 | 0.75 |
| Body mass index, kg/m2* | 27±4 | 25±4 | 0.27 |
| Etiology, % | |||
| Dilated cardiomyopathy | 42 | 61 | 0.12 |
| Ischemic cardiomyopathy | 53 | 27 | 0.02 |
| Other | 5 | 12 | 0.38 |
| Diabetes therapy, % | |||
| Oral hypoglycemics | 32 | – | – |
| Insulin | 44 | – | – |
| Diabetic diet | 32 | – | – |
| Serum glucose (random), mmol/L* | 13.0±7.0 | 6.2±1.1 | <0.001 |
| LDL cholesterol, mmol/L* | 3.0±1.7 | 2.5±1.0 | 0.15 |
| HDL cholesterol, mmol/L* | 0.80±0.32 | 0.92±0.35 | 0.24 |
| Hemoglobin, g/L* | 138±22 | 130±21 | 0.24 |
| Leukocytes, × 109/L* | 9.1±4.3 | 8.2±2.2 | 0.22 |
| Thrombocytes, × 109/L* | 197±51 | 232±80 | 0.14 |
| Serum AST, U/L* | 40±30 | 33±17 | 0.28 |
| Serum ALT, U/L* | 30±16 | 34±23 | 0.65 |
| Serum bilirubin, μmol/L* | 26±13 | 23±11 | 0.43 |
| Serum creatinine, μmol/L* | 135±49 | 153±95 | 0.44 |
| Serum urea, mmol/L* | 15±8 | 14±10 | 0.66 |
Data presented as mean ± SD. ALT Alanine aminotransferase; AST Aspartate aminotransferase; HDL High-density lipoprotein; LDL Low-density lipoprotein
Postoperatively, diabetic and nondiabetic cardiac transplant recipients received similar immunosuppression regimens. In both groups, the majority of patients received a triple-therapy combination of cyclosporine, mycophenolate mofetil and tapering doses of prednisone. In a small number of patients, either tacrolimus or sirolimus was exchanged for cyclosporine. Throughout the three-year follow-up period, diabetic and nondiabetic cardiac transplant recipients had no difference in their serum cyclosporine and tacrolimus levels, and no difference in their prednisone and mycophenolate mofetil doses.
Table 2 summarizes the results of routine blood work measured at three months, and one, two and three years after transplantation. Despite similar serum creatinine levels in diabetic and nondiabetic cardiac transplant recipients at the three-month follow-up examination, diabetic patients had significantly higher serum creatinine levels at three years following transplantation (211±180 μmol/L versus 140±42 μmol/L; P<0.01). However, when serum creatinine was corrected for age, weight and sex using the Cockcroft-Gault equation, there was no difference between groups with respect to creatinine clearance, at both one and three years after transplantation. Additionally, at two-year follow-up, diabetic recipients had increased serum urea compared with the nondiabetic group (13.2±5.3 mmol/L versus 10.2±3.7 mmol/L; P=0.01). Table 2 also demonstrates similar low-density lipoprotein cholesterol values for both groups throughout the three-year follow-up period, although diabetic recipients showed a decreased high-density lipoprotein cholesterol level at three years (1.03±0.20 mmol/L versus 1.31±0.43 mmol/L; P=0.04).
TABLE 2.
Routine follow-up blood work in diabetic and nondiabetic cardiac transplant recipients
| Three-month follow-up | Diabetic patients | Nondiabetic patients | P |
|---|---|---|---|
| Hemoglobin, g/L | 111±15 | 116±17 | 0.45 |
| Leukocytes, × 109/L | 7.9±2.8 | 9.1±9.7 | 0.65 |
| Thrombocytes, × 109/L | 258±83 | 264±67 | 0.75 |
| Serum AST, U/L | 23±8 | 24±9.3 | 0.57 |
| Serum ALT, U/L | 22±10 | 20±11 | 0.69 |
| Serum bilirubin, μmol/L | 18.9±6.0 | 18.0±7.1 | 0.70 |
| Serum creatinine, μmol/L | 145±42 | 143±74 | 0.97 |
| Serum urea, mmol/L | 15.0±6.7 | 12.3±10.3 | 0.40 |
|
One-year follow-up | |||
| Hemoglobin, g/L | 130±19 | 128±15 | 0.69 |
| Leukocytes, × 109/L | 6.5±2.3 | 6.9±6.2 | 0.85 |
| Thrombocytes, × 109/L | 202±69 | 228±67 | 0.23 |
| Serum AST, U/L | 23±7 | 24±8 | 0.87 |
| Serum ALT, U/L | 19±8 | 20±10 | 0.84 |
| LDL cholesterol, mmol/L | 3.0±0.9 | 2.6±1.0 | 0.41 |
| HDL cholesterol, mmol/L | 1.10±0.37 | 1.38±0.48 | 0.11 |
| Serum bilirubin, μmol/L | 16.9±5.7 | 18.1±6.9 | 0.61 |
| Serum creatinine, μmol/L | 158±56 | 151±101 | 0.83 |
| Creatinine clearance, mL/s | 1.02±0.39 | 1.00±0.46 | 0.91 |
| Serum urea, mmol/L | 13.0±4.6 | 11.4±5.2 | 0.29 |
|
Two-year follow-up | |||
| Hemoglobin, g/L | 127±20 | 132±15 | 0.25 |
| Leukocytes, × 109/L | 6.7±2.0 | 6.8±2.1 | 0.96 |
| Thrombocytes, × 109/L | 189±56 | 223±55 | 0.05 |
| Serum AST, U/L | 21±7 | 27±15 | 0.19 |
| Serum ALT, U/L | 17±7 | 23±22 | 0.38 |
| Serum bilirubin, μmol/L | 14.9±4.6 | 17.3±6.9 | 0.26 |
| Serum creatinine, μmol/L | 179±95 | 147±80 | 0.2 |
| Serum urea, mmol/L | 13.2±5.3 | 10.2±3.7 | 0.01 |
|
Three-year follow-up | |||
| Hemoglobin, g/L | 128±913 | 133±15 | 0.25 |
| Leukocytes, × 109/L | 6.7±2.1 | 6.3±1.9 | 0.51 |
| Thrombocytes, × 109/L | 178±85 | 219±55 | 0.03 |
| Serum AST, U/L | 21±67 | 22±6 | 0.71 |
| Serum ALT, U/L | 19±6 | 22±27 | 0.69 |
| LDL cholesterol, mmol/L | 2.8±0.5 | 2.5±0.8 | 0.33 |
| HDL cholesterol, mmol/L | 1.03±0.20 | 1.31±0.43 | 0.04 |
| Serum bilirubin, μmol/L | 14.4±4.8 | 16.0±4.6 | 0.26 |
| Serum creatinine, μmol/L | 211±180 | 140±42 | <0.01 |
| Creatinine clearance, mL/s | 0.91±0.40 | 1.02±0.47 | 0.44 |
| Serum urea, mmol/L | 12.7±3.1 | 11.1±7.0 | 0.43 |
Data presented as mean ± SD. ALT Alanine aminotransferase; AST Aspartate aminotransferase; HDL High-density lipoprotein; LDL Low-density lipoprotein
Serum glucose levels and diabetic therapy requirements are displayed in Tables 3 and 4, respectively. Diabetic transplant recipients had significantly higher (P<0.001) serum glucose levels throughout the three-year follow-up period. Consequently, a significantly larger proportion of diabetic patients received oral hypoglycemics and insulin (P<0.01).
TABLE 3.
Follow-up fasting serum glucose in diabetic and nondiabetic cardiac transplant recipients
| Serum glucose, mmol/L, mean ± SD | Diabetic patients | Nondiabetic patients | P |
|---|---|---|---|
| At one-year follow-up | 9.0±3.1 | 5.6±1.0 | <0.001 |
| At two-year follow-up | 9.0±3.6 | 5.8±1.0 | <0.001 |
| At three-year follow-up | 9.0±4.7 | 6.0±1.4 | <0.001 |
TABLE 4.
Percentage of diabetic and nondiabetic cardiac transplant recipients requiring diabetic therapy preoperatively and at postoperative follow-up
| Diabetic patients, % (n=19) | Nondiabetic patients, % (n=117) | P | |
|---|---|---|---|
| Preoperatively | |||
| Oral hypoglycemics | 32 | – | – |
| Insulin | 44 | – | – |
| Diabetic diet only | 32 | – | – |
| Three months postoperatively | |||
| Oral hypoglycemics | 31 | 2 | <0.001 |
| Insulin | 77 | 3 | <0.001 |
| Diabetic diet only | 8 | 1 | 0.12 |
| One year postoperatively | |||
| Oral hypoglycemics | 31 | 1 | <0.001 |
| Insulin | 62 | 2 | <0.001 |
| Diabetic diet only | 8 | 2 | 0.30 |
| Two years postoperatively | |||
| Oral hypoglycemics | 31 | 2 | <0.001 |
| Insulin | 70 | – | – |
| Diabetic diet only | 8 | 1 | 0.13 |
| Three years postoperatively | |||
| Oral hypoglycemics | 25 | 3 | 0.002 |
| Insulin | 75 | 0 | <0.001 |
| Diabetic diet only | 8 | 3 | <0.001 |
Diabetic and nondiabetic transplant recipients showed similar rates of clinically significant infection (53% versus 40%; P=0.29) and rejection (26% versus 15%; P=0.20) during the first three postoperative months.
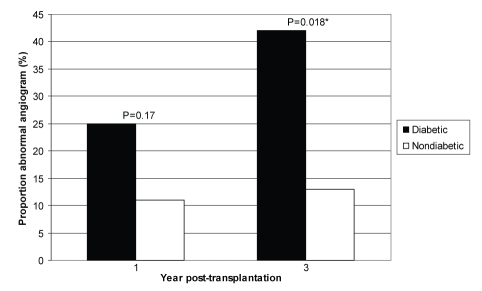
Table 5 and Figure 1 show the results of coronary angiography performed at one- and three-year follow-up examinations. As discussed above, TCAD was defined as any luminal irregularity or more severe lesion on angiography. One year after transplantation, diabetic and non-diabetic transplant recipients showed no difference in the rate of coronary artery disease (25% versus 11% had an abnormal angiogram; P=0.17). However, three years after transplantation, diabetic recipients showed a significantly increased rate of abnormal angiograms (42% versus 13%; P=0.02).
TABLE 5.
Results of abnormal cardiac angiograms in diabetic and nondiabetic cardiac transplant recipients at one and three years after cardiac transplantation
|
Diabetic recipients (n=19) | |
| One year post-transplant (25%) | RCA: mild irregularity |
| RCA: >80% stenosis; LAD: mild irregularity distally | |
| LAD: 50% stenosis distally | |
| Three years post-transplant (42%) | Diagonal artery: mild irregularity |
| RCA: mild irregularity | |
| LAD: mid/distal ectasia; CX: proximal ectasia | |
| RCA, LAD, CX: mild irregularities | |
| RCA: mild irregularity, and diffuse, severe narrowing and occlusion in the PD and PL; LAD: diffuse, severe narrowing distally with small branch occlusion; CX: occluded distally; obtuse marginal artey: ectasia proximally with midsegment 40% to 50% stenosis | |
|
Nondiabetic recipients (n=117) | |
| One year post-transplant (11%) | PL, RI: ectasia distally |
| RCA, LAD: ectasia proximally | |
| RCA, LAD, CX: mild irregularity | |
| LAD: 30% stenosis | |
| LAD: occluded distally with diffusely narrowed, occluded septal branches; CX: diffusely narrowed; Diagonal artery: diffusely diseased; RI: occluded proximally; Marginal artery: 80% to 90% stenosis | |
| RCA: diffuse, severe narrowing in branches; LAD: diffuse, severe narrowing distally; CX, diagonal artery: diffuse narrowing | |
| LAD: 30% ostial stenosis, diffuse mild irregularity | |
| RCA: 30% stenosis | |
| LAD, CX: diffuse, severe narrowing; PD: irregularity | |
| Three years post-transplant (13%) | RCA: diffuse irregularity, ectasia; LAD, CX: diffuse irregularity, multiple areas of ectasia |
| RCA: midsegment irregularity; RI: 30% to 40% stenosis | |
| RCA, LAD: proximal ectasia | |
| RCA: mild irregularity proximally; LAD, CX: mild irregularity; PL, PD: diffuse irregularity | |
| RCA, LAD, CX: 30% stenosis | |
| Diffuse mild irregularities | |
| RCA: 40% stenosis | |
| RCA: 40% stenosis | |
Percentages in parentheses represent the percentage of abnormal angiograms. Results are organized as the individual results of abnormal angiograms in each category. CX Circumflex artery; LAD Left anterior descending artery; PD Posterior descending artery; PL Posterolateral artery; RCA Right coronary artery; RI Ramus intermedius
Figure 1).
Proportion of diabetic and nondiabetic cardiac transplant recipients with abnormal angiograms one and three years after transplantation. *Significant difference
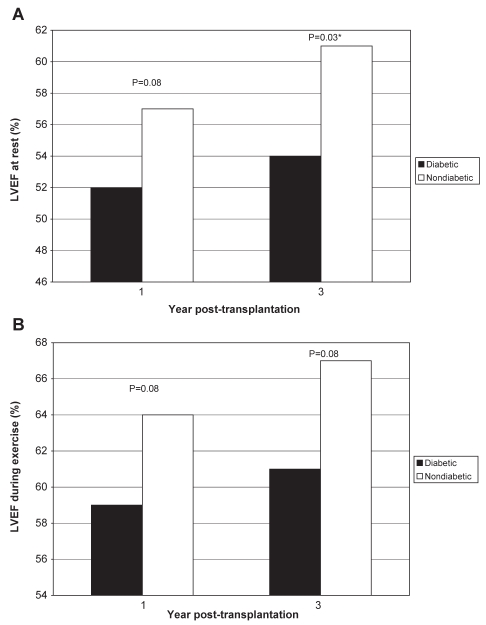
Results of the nuclear medicine studies performed at one and three years post-transplantation are summarized in Figure 2. LVEF, as measured by a nuclear medicine study, was similar between diabetic and nondiabetic patients at one-year follow-up at rest (52±20% versus 57±11%, respectively; P=0.08) and during exercise (59±6% versus 64±10%, respectively; P=0.08). At three years post-transplantation, LVEF measured at rest was significantly lower in diabetic recipients (54±11% versus 61±9%; P=0.03). However, differences in LVEF during exercise between diabetic and nondiabetic patients did not reach statistical significance (61±10% versus 67±9%, respectively; P=0.08).
Figure 2).
A Left ventricular ejection fraction (LVEF) in diabetic and nondiabetic cardiac transplant recipients at rest at one and three years after transplantation. B LVEF in diabetic and nondiabetic cardiac transplant recipients during supine bicycle exercise at one and three years after transplantation. *Significant difference
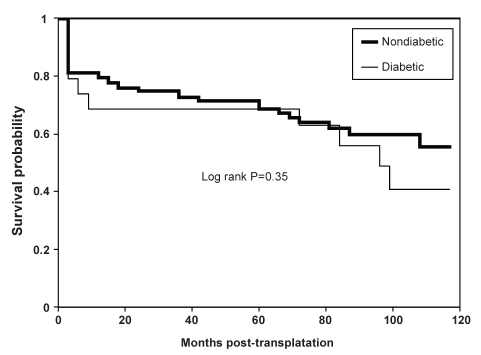
Finally, Kaplan-Meier analysis (Figure 3) revealed similar rates of actuarial survival in diabetic and nondiabetic cardiac transplant recipients up to 10 years after transplantation (log-rank P=0.35).
Figure 3).
Actuarial survival curves for diabetic and nondiabetic cardiac transplant recipients calculated by Kaplan-Meier regression analysis. Log-rank P=0.35
DISCUSSION
The present retrospective single-centre study demonstrates increased rates of morbidity in cardiac transplant recipients with preoperative diabetes mellitus. Specifically, diabetic recipients revealed increased rates of TCAD and decreased cardiac function three years after transplantation. Although serum creatinine was elevated in diabetic recipients, there were no differences in creatinine clearance. Interestingly, diabetic and nondiabetic patients showed no differences in rates of clinically significant infection or rejection during the first three postoperative months. Furthermore, survival rates were similar between these two groups.
Nephropathy is a well-known complication of diabetes mellitus and a source of increased concern when considering diabetic cardiac patients for transplantation. In the present study, serum creatinine and creatinine clearance were used to monitor renal function. Before transplantation, diabetic and nondiabetic recipients demonstrated similar creatinine levels. Renal function remained comparable between these two groups during the initial follow-up period. However, three years after transplantation, diabetic transplant recipients showed significantly increased serum creatinine levels. This result is in contrast to at least four previous studies (6,7,12,16) that demonstrated no difference in serum creatinine at an intermediate-term follow-up period of either three or five years. A more recent study (13), which included 243 patients, noted significant renal impairment in diabetic recipients in as early as 12 months. Furthermore, a recent review of the United Network of Organ Sharing (UNOS) database demonstrated improved renal failure-free survival in nondiabetic transplant recipients (15). However, in our study, when serum creatinine was corrected for age, weight and sex, the two groups showed similar renal function throughout the three-year follow-up. At our centre, every attempt is made to decrease the dose of calcineurin inhibitors for all patients demonstrating renal dysfunction. We do not systematically switch all of our diabetic patients, or those with renal dysfunction, to sirolimus. Urine microalbumin is now being measured routinely at our centre. This may allow earlier detection of renal compromise, and demonstrate increased discrepancies between diabetic and nondiabetic transplant recipients in future studies.
A recent investigation (17) confirmed that poor glucose control in diabetic patients is associated with an increased rate of postoperative infections. This concern is further amplified in heart transplantation because of the mandatory immunosuppressive regimen required after surgery. A recent review of the UNOS database (15) revealed improved infection-free survival in nondiabetic recipients. However, most previous studies failed to recognize any difference in the rates of infection in diabetic and nondiabetic cardiac transplant recipients (6–13). In our study, diabetic and nondiabetic transplant recipients showed similar rates of infection requiring antibiotics or hospitalization in the first three postoperative months. Within these first three months, our study also showed similar rates of rejection between the two groups. Similarly, previous investigations have failed to demonstrate a difference in the rates of rejection between diabetic and non-diabetic recipients (6–13,15,16).
Diabetes is a well-recognized risk factor for the development of cardiovascular disease, resulting in a two- to fourfold increase in coronary artery disease (18). Therefore, it seems reasonable to predict an increased incidence of TCAD in diabetic versus nondiabetic cardiac transplant recipients. Interestingly, previous studies, including a review of more than 20,000 patients in the UNOS database, have failed to recognize such a difference (6,8,10–16). In our investigation, the tendency for increased TCAD in diabetic recipients one year after surgery was not statistically significant. However, our study revealed angiographic abnormalities indicative of TCAD in a significantly larger proportion of the diabetic cardiac transplant recipients at three-year follow-up. Low-density lipoprotein cholesterol levels were elevated beyond 2.5 mmol/L in both groups throughout the follow-up period, but there was no significant difference between the groups. However, the decreased high-density lipoprotein cholesterol in the diabetic recipients three years after transplantation may suggest the loss of a protective factor, contributing to the increased incidence of TCAD.
The current study is the first to investigate differences in cardiac function in diabetic and nondiabetic cardiac transplant recipients. LVEF was similar between the two groups at the one-year follow-up. However, three years after transplantation, diabetic recipients showed a significantly lower ejection fraction on a wall motion study while at rest, but not during stress.
The majority of previous studies have shown no significant differences in short- and long-term survival between diabetic and nondiabetic cardiac transplant recipients (6–11,16). A review of the UNOS database (15) showed no difference in survival between nondiabetic patients and patients with uncomplicated diabetes. One study (14) demonstrated decreased 10-year survival rates in diabetic versus non-diabetic recipients. A more recent study (13) revealed decreased one-year survival in diabetic recipients of cardiac transplantation between 1986 and 1994, but not between 1995 and 2003. In our investigation, Kaplan-Meier analysis revealed similar rates of actuarial survival in diabetic and nondiabetic recipients, even at 10-year follow-up. This suggests that the increased postoperative morbidity demonstrated in diabetic cardiac transplant recipients during the first three years does not adversely affect intermediate-term survival. However, increased follow-up is necessary to determine any differences in long-term survival between diabetic and nondiabetic recipients.
There were two major limitations to our investigation. The first was the limited sample of diabetic patients available for the study. However, despite our small population of diabetic cardiac transplant recipients, we were able to observe two key statistically significant results, suggesting the need for a large multicentre survey to fully assess the implications of diabetes in cardiac transplant recipients. The second limitation was that this was a retrospective study. However, multiple databases were reviewed in an attempt to avoid incomplete and inaccurate data.
To the best of our knowledge, the current study is the first to demonstrate that preoperative diabetes is associated with an increased risk of TCAD, as well as decreased cardiac function, as early as three years after cardiac transplantation. However, despite this increased morbidity associated with pretransplant diabetes, the survival rate remains similar for diabetic and nondiabetic cardiac transplant recipients. We suggest that improved postoperative diabetic control will result in better outcomes for this population.
CONCLUSION
Uncomplicated diabetes mellitus is not a contraindication to cardiac transplantation, but increased vigilance is warranted in this population to minimize postoperative morbidity.
REFERENCES
- 1.Harris SB, Lank CN, Capes SE, et al. Canadian Diabetes Association 2003 clinical practice guidelines for the prevention and management of diabetes in Canada. Can J Diabetes. 2003;27(Suppl 2):S1–152. [Google Scholar]
- 2.Kannel WB, McGee DL. Diabetes and cardiovascular disease: The Framingham study. JAMA. 1979;241:2035–8. doi: 10.1001/jama.241.19.2035. [DOI] [PubMed] [Google Scholar]
- 3.Maxey TS, Keeling WB, Sommers KE. Surgical alternatives for the palliation of heart failure: A prospectus. J Card Fail. 2005;11:670–6. doi: 10.1016/j.cardfail.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 4.Steinman TI, Becker BN, Frost AE, et al. Guidelines for the referral and management of patients eligible for solid organ transplantation. Transplantation. 2001;71:1189–204. doi: 10.1097/00007890-200105150-00001. [DOI] [PubMed] [Google Scholar]
- 5.Ross H, Hendry P, Dipchand A, et al. 2001 Canadian Cardiovascular Society Consensus Conference on Cardiac Transplantation. Can J Cardiol. 2003;19:620–54. [PubMed] [Google Scholar]
- 6.Czerny M, Sahin V, Zuckerman A, et al. Diabetes affects long-term survival after heart transplantation. J Heart Lung Transplant. 2001;20:245. [PubMed] [Google Scholar]
- 7.Lang CC, Beniaminovitz A, Edwards N, Mancini DM. Morbidity and mortality in diabetic patients following cardiac transplantation. J Heart Lung Transplant. 2003;22:244–9. doi: 10.1016/s1053-2498(02)00567-3. [DOI] [PubMed] [Google Scholar]
- 8.Munoz E, Lonquist JL, Radovancevic B, et al. Long-term results in diabetic patients undergoing heart transplantation. J Heart Lung Transplant. 1992;11:943–9. [PubMed] [Google Scholar]
- 9.Morgan JA, John R, Weinberg AD, Colletti NJ, Mancini DM, Edwards NM. Heart transplantation in diabetic recipients: A decade review of 161 patients at Columbia Presbyterian. J Thorac Cardiovasc Surg. 2004;127:1486–92. doi: 10.1016/j.jtcvs.2003.11.063. [DOI] [PubMed] [Google Scholar]
- 10.Rhenman MJ, Rhenman B, Icenogle T, Christensen R, Copeland J. Diabetes and heart transplantation. J Heart Transplant. 1988;7:356–8. [PubMed] [Google Scholar]
- 11.Ladowski JS, Kormos RL, Uretsky BF, Griffith BP, Armitage JM, Hardesty RL. Heart transplantation in diabetic recipients. Transplantation. 1990;49:303–5. doi: 10.1097/00007890-199002000-00015. [DOI] [PubMed] [Google Scholar]
- 12.Livi U, Caforio AL, Grassi G, et al. Mid-term results of heart transplantation in diabetic recipients. J Cardiovasc Surg. 1994;35(6 Suppl 1):115–8. [PubMed] [Google Scholar]
- 13.Klingenberg R, Gleissner C, Kock A, et al. Impact of pre-operative diabetes mellitus upon early and late survival after heart transplantation: A possible era effect. J Heart Lung Transplant. 2005;24:1239–46. doi: 10.1016/j.healun.2004.09.007. [DOI] [PubMed] [Google Scholar]
- 14.Czerny M, Sahin V, Fasching P, et al. The impact of diabetes mellitus at the time of heart transplantation on long-term survival. Diabetologia. 2002;45:1498–508. doi: 10.1007/s00125-002-0960-0. [DOI] [PubMed] [Google Scholar]
- 15.Russo MJ, Chen JM, Hong KN, et al. Survival after heart transplantation is not diminished among recipients with uncomplicated diabetes mellitus: An analysis of the United Network of Organ Sharing database. Circulation. 2006;114:2280–7. doi: 10.1161/CIRCULATIONAHA.106.615708. [DOI] [PubMed] [Google Scholar]
- 16.Marelli D, Laks H, Patel B, et al. Heart transplantation in patients with diabetes mellitus in the current era. J Heart Lung Transplant. 2003;22:1091–7. doi: 10.1016/s1053-2498(02)01219-6. [DOI] [PubMed] [Google Scholar]
- 17.Dronge AS, Perkal MF, Kancir S, Concato J, Aslan M, Rosentahal RA. Long-term glycemic control and post-operative infectious complications. Arch Surg. 2006;141:375–80. doi: 10.1001/archsurg.141.4.375. [DOI] [PubMed] [Google Scholar]
- 18.Feskens EJ, Kromhout D. Glucose tolerance and the risk of cardiovascular disease: The Zutphen Study. J Clin Epidemiol. 1992;45:1327–34. doi: 10.1016/0895-4356(92)90173-k. [DOI] [PubMed] [Google Scholar]