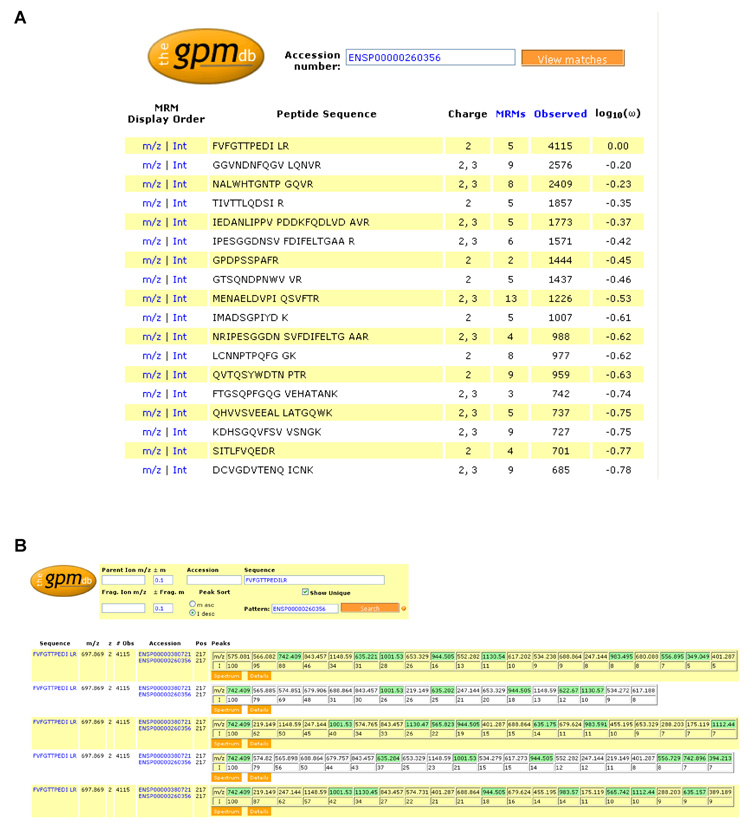
Figure 2. The GPMDB MRM worksheet.
Screen shots from the newly-developed worksheet for the design of MRM transitions at the http://gpmdb.thegpm.org. A. shows the MRM worksheet for the protein thrombospondin-1 (THBS1, swissprot accession number P07996), listing the peptide sequence, charge state the peptide has been observed in, the number of consensus spectra available for each peptide sequence across all charge states, sequence modifications, and instrument types (denoted as “MRMs” in the worksheet to appropriately reflect its use), the number of observations of the peptide in the database and the frequency of observation (log10(ω)). B. shows in detail the summary page displaying the 5 sets of MRMs predicted for the most observed thrombospondin-1 peptide (FVFGTTPEDILR), based on its 5 consensus spectra. The peptide sequence and occurring post-translational modification sites (indicated by the blue background and display of the associated mass shift when locating the mouse pointer on this position), m/z ratio, charge state and number of observations in the GPMDB of the precursor ion are listed, as well as the m/z ratio and relative intensities of the fragment ions. Unique transitions are highlighted in green. Note that the information in these screen shots was current as of 28th August 2008, but as the GPMDB is a dynamic, living database, certain information, such as the number of observations of a peptide, are likely to change with time as the database grows.