Abstract
Circadian clocks organize a wide array of metabolic functions in a coherent daily schedule and ensure synchrony of this schedule with environmental rhythms. Daily rhythmicity of lipid metabolism occurs in rodents and ruminants. We examined daily level variations of serum lipids (nonesterified fatty acids [NEFA], triglycerides, phospholipids, total cholesterol and total lipids) in healthy dogs, particularly focusing on their temporal relationship to lighting and fasting cycles. Whereas serum NEFA levels did not change across the day, levels of total lipids, total cholesterol, phospholipids, and triglycerides occurred in dogs maintained under 12:12-h light:dark cycles and fed a single meal daily. Only the rhythmic pattern of triglycerides responded to a 6 h delay in light onset, suggesting a cardinal role of a light-entrained circadian oscillator in its generation. To investigate whether temporal variations in serum lipids depend to physiological postprandial changes, we measured lipid levels in fasted dogs. Rhythms of total lipids, total cholesterol, phospholipids, and triglycerides vanished when dogs were food-deprived, indicating that these rhythms are driven by the digestive process. Levels of serum NEFA patterns were significantly higher during fasting than after food intake. The increase of NEFA concentrations during fasting may reflect the mobilization of adipose tissue NEFA mediated by the decrease in insulin with its lypolitic effects. Elucidating the daily rhythmicity of lipid levels is fundamental to understanding the metabolism of the dog, an animal model frequently used for research in metabolic pathophysiology.
Abbreviations: NEFA, nonesterified fatty acids
Circadian clocks are autonomous internal daily timekeeping mechanisms that allow organisms to adapt to external daily rhythms of many environmental factors.31 The adaptive importance of circadian timekeeping has been illustrated in several model systems.10 Fundamental properties of circadian oscillators are that they produce a rhythm with a free-running period that can be synchronized by periodic environmental cues.27,32 The most pervasive environmental signals for the mammalian circadian system are the natural light–dark cycle and food availability. Photic entrainment is entirely dependent on light input through the eyes to the suprachiasmatic nuclei of the anterior hypothalamus. Bilateral eye-enucleated mammals do not show any behavioral entrainment by light–dark cycles.40 Because feeding entrainment persists in animals with lesions of the suprachiasmatic nucleus,26,36-38 it may be regulated by a different circadian system. The dorsomedial nucleus of the hypothalamus recently was reported to play an important role in feeding entrainment.15
The circadian clock reportedly regulates metabolism and energy homeostasis in the liver and other peripheral tissues by mediating the expression or activity of various metabolic enzymes and transport systems involved in cholesterol, glycogen, and glucose metabolism.8,17,24,43 The importance of circadian timekeeping for metabolism is shown clearly by the metabolic phenotypes associated with mice carrying mutations in clock genes.22,35,39
Daily rhythmicity of lipid metabolism has been described in different mammalian orders. For instance, the plasma concentrations of nonesterified fatty acids (NEFA), triglycerides and cholesterol displayed daily variations in rodents13,22, and ruminants.1,5,29,42
Elucidating the daily rhythmicity of lipid levels is a fundamental necessity for understanding the metabolism of the dog, an animal model frequently used for research in the pathophysiology of diabetes mellitus. However, circadian investigations of the metabolism of dogs have been scarce. Previous investigations showed diurnal variations of various metabolic and neuroendocrine activities in dogs in association with feeding–fasting cycles.16,19 However, these studies did not use canonical experimental protocols, which are necessary for the identification of rhythmicity.
Here we examined daily level variations of serum lipids (nonesterified fatty acids [NEFA], triglycerides, phospholipids, total cholesterol, and total lipids) in healthy dogs, particularly focusing on their temporal relationship to lighting and fasting cycles. The goals of this study were to determine whether levels of these plasma lipids show circadian variation, and, if so, whether the variations related to the lighting cycles, reflect simple postprandial changes, or are under the control of a circadian clock.
Materials and Methods
Animals and housing.
Five clinically healthy purebred Beagles (Canis familiaris, male, 2 y old, mean body mass = 13 kg) were used. During tests the animals were housed in individual pens (140 × 200 cm) equipped with a darkened opening and an airflow system. The visual and acoustic isolation of each dog from conspecifics prevented social entrainment of circadian behavioral rhythms.9 Animal room temperature and relative humidity ranges were 21 ± 2 °C and 40% to 60%, respectively. Thermal and hygrometric recordings in each pen throughout the study were obtained by means of a data logger (TH2500, Gemini Tinytag, Dundee, Scotland). Lighting was diffused uniformly throughout animal facilities and provided sufficient illumination to aid in maintaining good housekeeping practices, adequate inspection of animals, safe working conditions for personnel, and the wellbeing of the animals. Dogs were kept under artificial light-dark cycles (light phase, 800 lx at the level of the dogs’ heads; dark phase, 0.1 lx). Light was provided by cool daylight fluorescent tubes (FH HE/860 Lumilux T5, Osram GmbH, Munich, Germany) placed in the middle of the pen at a height of 2 m from the floor. The light intensity was measured by a photometer (PCE172, PCE Group, Lucca, Italy). A dim red light (< 3 lx, 15 W; Safelight lamp filter 1A, Kodak Spa, Milano, Italy) was used to feed and sample dogs during the dark phase.
A certified dog diet (Teklad 2021 Global Dog, Harlan Laboratory, Udine, Italy) was provided to each animal (approximately 270 g daily; crude protein, 21.2%; crude oil, 6.1%; crude fiber, 4.6%; ash, 6.9%; moisture, 6.6%; nitrogen-free extract, 51.2%; carbohydrate, 52.6%; starch, 34.4%; sugar, 5.4%; saturated fatty acid, 12.7 g/kg; monounsaturated fatty acid, 18.5 g/kg; polyunsaturated fatty acid, 28.8 g/kg; digestible energy, 3.4 kcal/g; metabolizable energy, 3.2 kcal/g) at 1000 each day. Water was supplied ad libitum.
All treatments, housing and care were carried out under guidelines for the care and use of laboratory animals established by the Italian Ministry of Health and the European Union14,20
Experimental design.
Blood sampling was carried out on dogs exposed to a 12:12-h light:dark cycle, with lights on at 0900 (LD1). Samples were withdrawn every 4 h over a 24-h period from 0900; dim red lighted was used as needed for sampling during dark phases. The day after blood sampling, dogs were exposed to a 6-h delay in light onset (LD2; lights on at 1500). Fifteen days later, blood samples were drawn from dogs every 4 h for 24 h beginning at 0900. After a recovery period of 2 wk, dogs were deprived of food for an entire day before blood sampling. The next day, blood samples were withdrawn every 4 h for 24 h beginning at 0900; food was withheld on the day of sampling.
Assays.
Dogs were restrained manually, and blood samples (5 ml) were collected from the cephalic vein by using collection tubes without anticoagulant (21-gauge × 1-in. needle; Vacutest 11030, Vacutest Kima, Padova, Italy). Bleeding was stopped before the dog was returned to its pen by applying finger pressure to the sampling site for approximately 30 s. Potential stress associated with the bleeding was minimized by training and acclimating dogs to restraint. After clotting at room temperature for 1 h, blood samples were centrifuged (4235 A, ALC, Milano, Italy) at 3000 × g for 20 min and the obtained sera were stored at −20 °C until being assayed. Serum lipids assays were carried out by means of a UV spectrophotometer (DU40, Beckman Instruments, Fullerton, CA) by using standard methods and procedures.18 Total lipids were determined by means an enzymatic colorimetric test based on sulphophosphovainilline reactivity (catalog no. 25195, Lickson, Palermo, Italy). Triglycerides, phospholipids, and total cholesterol were determined after enzymatic hydrolysis by means of an enzymatic colorimetric test (GPO-PAP method). Briefly, triglycerides were determined after enzymatic hydrolysis with lipoprotein lipase. The indicator was a colored phenazone formed from hydrogen peroxide, 4-aminoantipyrine, and 4-chlorophenol under the catalytic influence of peroxidase (Cat. number 1000100, Centronic GmbH, Wartenberg, Germany). Phospholipids were determined after enzymatic hydrolysis with phospholipase D (catalog no. 25140, Centronic GmbH). Total cholesterol was determined after enzymatic hydrolysis and oxidation. Hydrogen peroxide created formed a red dyestuff by reacting with 4-aminoantipyrine in the presence of phenol and peroxidase. The color intensity is directly proportional to the concentration of cholesterol (catalog no. CL06000030, Centronic GmbH). NEFA were measured enzymatically with a commercially available kit (catalog no. FA115; Randox Laboratories, Crumlin, United Kingdom). All samples were analyzed in duplicate. Samples exhibited parallel displacement to the standard curve; the intraassay coefficient of variation was less than 8%.
Statistical analysis.
All results are expressed as mean ± SEM. To compare overall levels of parameters in the different tests, mean levels over a daily period were compared. Either 1- or 2-way repeated-measures ANOVA was used to determine significant differences, and P values less than 0.05 were considered statistically significant. The Bonferroni test was applied for post hoc comparison. Data were analyzed by using the software Statistica 5.5 (StatSoft, Tulsa, OK). In addition, we applied a trigonometric statistical model to the average values of each time series, so as to describe the periodic phenomenon analytically, by individuating the main rhythmic parameters according to the single cosinor procedure.28 Three rhythmic parameters were determined: acrophase, amplitude, and robustness (strength of rhythmicity). Acrophase was the time of day at which the peak of a rhythm occurred. The amplitude of a rhythm was calculated as half the range of oscillation, which in its turn was computed as the difference between peak and trough. Rhythm robustness was computed as a percentage of the maximal score attained by the χ2 periodogram statistic for ideal data sets of comparable size and 24-h periodicity.33 Robustness greater than 70% is above noise level and indicates statistically significant rhythmicity.
Results
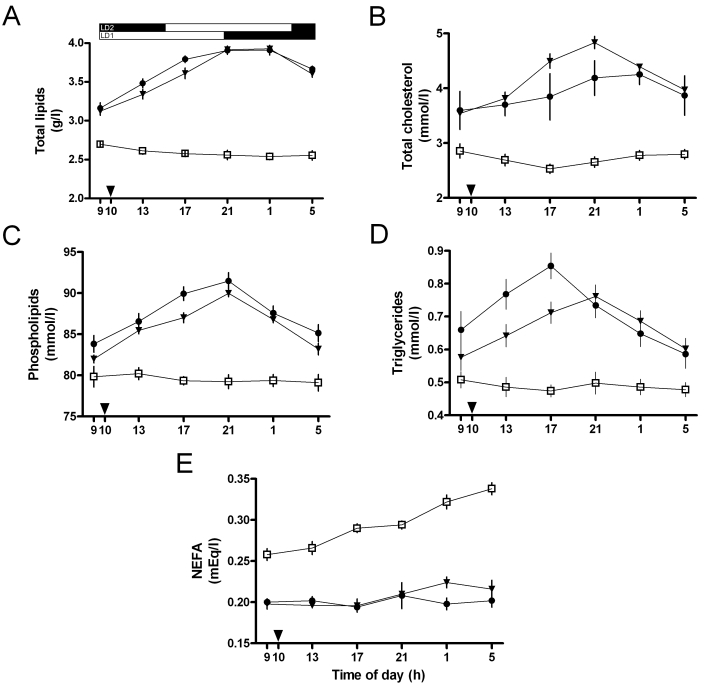
ANOVA of data from dogs subjected to 12:12-h light:dark cycles revealed a significant effect of time for all serum parameters tested (Table 1, Figure 1 A to D), with the exception of NEFA (Figure 1 E). Total lipids, total cholesterol, and phospholipids showed clear daily rhythms: troughs occurred at 0900, began to increase immediately after food administration (1000), reached acrophases after 11 h, before returning to basal levels (Table 1, Figure 1 A to C). Shifting the 12:12-h light:dark cycle by 6 h did not influence either the acrophase or robustness of all rhythms (Table 1; Figure 1 A to C).
Figure 1.
Serum (A) total lipids, (B) total cholesterol, (C) phospholipids, (D) triglycerides, and (E) NEFA in dogs maintained under 12:12-h light:dark cycles (LD1, lights on at 0900; LD2, lights on at 1500) and fed a single meal in the day (LD1, filled circles; LD2, filled triangles) or fasted (empty squares). Serum samples (n = 5 for each time point) were collected every 4 h for 24 h. Mean ± SEM at each time point is shown. White and black bars indicate the durations of light and dark phases, respectively. Arrowheads indicate the time of food administration (1000) in both LD cycles.
Triglycerides also showed a significant daily rhythm under 12:12-h light:dark cycles (Figure 1 D): levels increased before lights on and peaked in the middle of the light phase. During the dark phase, triglyceride levels decreased, and troughs occurred in the middle of the night. A 6-h delay of the 12:12-h light:dark cycle shifted the serum triglycerides acrophase by approximately 4 h (Figure 1 D). According to 2-way ANOVA, mean levels of total lipids, total cholesterol, phospholipids, and triglycerides did not differ between the 2 fed 12:12-h light:dark cycles (Table 1). In contrast, fasting of the dogs for 1 d significantly (P < 0.001) concentrations of these parameters and abolished their rhythms (Table 1, Figure 1 A to D). Serum NEFA concentrations were significantly (P < 0.0001) higher during fasting than after food intake (Figure 1 E).
Table 1.
Summary of the results
| Mean | SEM | F(5,20) | P | Robustness | Acrophase | Amplitude | ||
| Total lipids (g/l) | LD1 | 3.66 | 0.05 | 33.30 | <0.0001 | 84.8% | 22:23 | 0.39 |
| LD2 | 3.59 | 0.06 | 40.08 | <0.0001 | 91.8% | 22:38 | 0.40 | |
| F | 2.59 | 0.02 | 1.33 | >0.3 | ||||
| Total cholesterol (mmol/l) | LD1 | 3.91 | 0.13 | 17.15 | <0.0001 | 91.5% | 23:00 | 0.33 |
| LD2 | 4.17 | 0.09 | 19.29 | <0.0001 | 96.0% | 21:05 | 0.61 | |
| F | 2.72 | 0.04 | 1.52 | >0.2 | ||||
| Phospholipids (mmol/l) | LD1 | 87.42 | 0.61 | 30.04 | <0.0001 | 95.5% | 19:51 | 3.68 |
| LD2 | 85.74 | 0.52 | 45.16 | <0.0001 | 87.5% | 20:14 | 3.58 | |
| F | 79.99 | 0.36 | 0.2 | >0.9 | ||||
| Triglycerides (mmol/l) | LD1 | 0.71 | 0.02 | 50.54 | <0.0001 | 93.4% | 16:32 | 0.12 |
| LD2 | 0.66 | 0.02 | 73.46 | lt;0.0001 | 97.5% | 20:15 | 0.08 | |
| F | 0.49 | 0.01 | 0.37 | >0.8 | ||||
| NEFA (mEq/l) | LD1 | 0.2 | 0.01 | 0.27 | >0.9 | |||
| LD2 | 0.2 | 0.01 | 2.19 | >0.09 | ||||
| F | 0.29 | 0.01 | 18.8 | <0.0001 |
F, fasting; LD1, lights on at 0900, lights off at 2100; LD2, lights on at 1500, lights off at 0300
Discussion
Circadian clocks organize a wide array of metabolic functions in a coherent daily schedule and ensure the synchrony of this schedule with environmental rhythms.41 The importance of circadian timekeeping for metabolism is illustrated by metabolic phenotypes associated with mice carrying mutations for cardinal circadian regulators, such as Clock and Bmal1. Clock- and Bmal1-mutant mice show increased serum triglycerides concentrations and impaired gluconeogenesis.35,39 The molecular basis of these defects may lie in the abrogation of circadian transcription patterns of metabolic regulators.17,23
The present investigation revealed daily variations in the levels of selected serum lipids in dogs. In particular, blood concentration of total lipids, total cholesterol, phospholipids, and triglycerides showed robust daily rhythmicity in dogs maintained under 12:12-h light:dark cycles and fed a single meal daily. This finding is consistent with previous demonstrations of daily rhythmicity in serum lipids in rodents and ruminants.1,12,13,21,22,29 Total lipids, total cholesterol, and phospholipids in dogs did not respond to a 6-h delay in light onset, whereas the rhythmic pattern of triglycerides showed a marked shift of the acrophase. These data suggest that only serum triglyceride concentrations are regulated by a light-entrained circadian oscillator, which probably is located in the liver, the main site of serum triglycerides biosynthesis. Studies in Clock-mutant mice showed that molecular circadian clock controls metabolic functions such as triglyceride accumulation in liver and plasma.22,39 However, our investigation could not demonstrate the existence of an endogenous circadian clock generating serum triglyceride rhythm because this parameter was not measured under constant light or constant darkness.
The data show that serum NEFA levels in dogs do not change throughout the day. Previous research has documented daily variations in the serum NEFA of rodents and ruminants.1,2,13,22,43 These divergences in results could depend on the species studied. Dogs are monogastric, and their feeding behavior in captivity (1 large meal daily) differs from that of rodents (free access to food) and ruminants (prolonged digestive processes). Furthermore, synergisms between feeding and photic regimens in captivity could influence lipid metabolism.6,7,34,36,39
To investigate whether temporal variations in serum lipids depend on postprandial physiologic changes, we measured these parameters in fasted dogs. Our previous study showed that rhythmicity in body temperature and cardiovascular parameters is independent of the feeding schedule.30 Present results show that rhythms of total lipids, total cholesterol, phospholipids, and triglycerides vanish when in food-deprived dogs, indicating that these rhythms are driven by the digestive process. Furthermore, our data show that these parameters are lower in fasted dogs than in subjects fed a single meal daily. These findings confirm previous investigations in dogs.19
In contrast to other measures, serum NEFA pattern were higher during fasting than after food intake, as previously shown.12,13 Fasting is characterized by low glucose concentrations, and accordingly, low levels of insulin and high glucagon. NEFA release in response to fasting promotes maintenance of whole-body energy homeostasis in the absence of an external energy supply. The rapid increase in serum NEFA levels during fasting may reflect mobilization of NEFA from adipose tissue; this process is mediated by the decrease in insulin with its lypolitic effects.3,11
In summary, this study documents the existence of daily rhythms in serum lipid levels in healthy dogs and the influence of environmental variables such as lighting and feeding conditions on these levels. A main assumption of metabolic profile research is that abnormalities in parameters indicate disorders. However, blood metabolites are not always ideal indicators of metabolic and nutritional alterations because animals may modify their metabolism in response to fluctuations in metabolism within the physiologic range.4 Furthermore, previous and current results suggest that ignoring the diurnal variations of serum lipid levels could invalidate their diagnostic usefulness. The demonstration of daily variations in the serum lipid levels of various mammalian species1,16,25,38 indicates that sporadic sampling (daily, weekly, or monthly) may fail to reveal the true metabolic status. Our present data can inform future studies in which dogs are used as experimental models to investigate human metabolic syndromes.
Acknowledgments
This work was supported by research grants from the University of Messina (GP and FF) and the University of Ferrara (CB).
References
- 1.Alila-Johansson A, Eriksson L, Soveri T, Laakso M. 2004. Daily and annual variations of free fatty acid, glycerol, and leptin plasma concentrations in goats (Capra hircus) under different photoperiods. Comp Biochem Physiol A Mol Integr Physiol 138:119–131 [DOI] [PubMed] [Google Scholar]
- 2.Alila-Johansson A, Eriksson L, Soveri T, Laakso ML. 2006. The daily rhythms of melatonin and free fatty acids in goats under varying photoperiods and constant darkness. Chronobiol Int 23:565–581 [DOI] [PubMed] [Google Scholar]
- 3.Bailhache E, Nguyen P, Krempf M, Siliart B, Magot T, Ouguerram K. 2003. Lipoproteins abnormalities in obese insulin-resistant dogs. Metabolism 52:559–564 [DOI] [PubMed] [Google Scholar]
- 4.Bartley JC. 1989. Lipid metabolism and its diseases. : Kaneko JJ.editor Clinical biochemistry of domestic animals. San Diego: Academic Press; p 106–141 [Google Scholar]
- 5.Bitman J, Wood DL, Lefcourt AM. 1990. Rhythms in cholesterol, cholesteryl esters, free fatty acids, and triglycerides in blood of lactating dairy cows. J Dairy Sci 73:948–955 [DOI] [PubMed] [Google Scholar]
- 6.Dauchy RT, Blask DE, Sauer LA, Brainard GC, Krause JA. 1999. Dim light during darkness stimulates tumor progression by enhancing tumor fatty acid uptake and metabolism. Cancer Lett 144:131–136 [DOI] [PubMed] [Google Scholar]
- 7.Dauchy RT, Sauer LA, Blask DE, Vaughan GM. 1997. Light contamination during the dark phase in ‘photoperiodically controlled’ animal rooms: effect on tumor growth and metabolism in rats. Lab Anim Sci 47:511–518 [PubMed] [Google Scholar]
- 8.Davidson AJ, Castanon-Cervantes O, Stephan FK. 2004. Daily oscillations in liver function: diurnal vs circadian rhythmicity. Liver Int 24:179–186 [DOI] [PubMed] [Google Scholar]
- 9.Davidson AJ, Menaker M. 2003. Birds of a feather clock together sometimes: social synchronization of circadian rhythms. Curr Opin Neurobiol 13:765–769 [DOI] [PubMed] [Google Scholar]
- 10.DeCoursey PJ, Walker JK, Smith SA. 2000. A circadian pacemaker in free-living chipmunks: essential for survival? J Comp Physiol A 186:169–180 [DOI] [PubMed] [Google Scholar]
- 11.Desvergne B, Michalik L, Wahli W. 2006. Transcriptional regulation of metabolism. Physiol Rev 86:465–514 [DOI] [PubMed] [Google Scholar]
- 12.Dugan RE, Slakey LL, Briedis AV, Porter JW. 1972. Factors affecting the diurnal variation in the level of hydroxymethylglutaryl coenzyme A reductase and cholesterol-synthesizing activity in rat liver. Arch Biochem Biophys 152:21–27 [DOI] [PubMed] [Google Scholar]
- 13.Escobar C, Diaz-Munoz M, Encinas F, Aguilar-Roblero R. 1998. Persistence of metabolic rhythmicity during fasting and its entrainment by restricted feeding schedules in rats. Am J Physiol 274:R1309–R1316 [DOI] [PubMed] [Google Scholar]
- 14. European Economic Community. 1986. Council directive 86/609/CEE.
- 15.Gooley JJ, Schomer A, Saper CB. 2006. The dorsomedial hypothalamic nucleus is critical for the expression of food-entrainable circadian rhythms. Nat Neurosci 9:398–407 [DOI] [PubMed] [Google Scholar]
- 16.Goriya Y, Bahoric A, Marliss EB, Zinman B, Albisser AM. 1981. Diurnal metabolic and hormonal responses to mixed meals in healthy dogs. Am J Physiol 240:E54–E59 [DOI] [PubMed] [Google Scholar]
- 17.Green CB, Takahashi JS, Bass J. 2008. The meter of metabolism. Cell 134:728–742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hainline A, Jr, Karon J, Lippel K. 1983. Manual of laboratory operations. : Hainline A, Jr, Karon J, Lippel K, editors Lipid research clinics program, lipid and lipoprotein analysis. Washington (DC): Department of Health and Human Services, Public Health Service, National Institutes of Health [Google Scholar]
- 19.Ishioka K, Hatai H, Komabayashi K, Soliman MM, Shibata H, Honjoh T, Kimura K, Saito M. 2005. Diurnal variations of serum leptin in dogs: effects of fasting and refeeding. Vet J 169:85–90 [DOI] [PubMed] [Google Scholar]
- 20.Italian Ministry of Health. DL 27/1/1192 n.116
- 21.Jurevics H, Hostettler J, Barrett C, Morell P, Toews AD. 2000. Diurnal and dietary-induced changes in cholesterol synthesis correlate with levels of mRNA for HMG-CoA reductase. J Lipid Res 41:1048–1054 [PubMed] [Google Scholar]
- 22.Kudo T, Tamagawa T, Kawashima M, Mito N, Shibata S. 2007. Attenuating effect of clock mutation on triglyceride contents in the ICR mouse liver under a high-fat diet. J Biol Rhythms 22:312–323 [DOI] [PubMed] [Google Scholar]
- 23.Lamia KA, Storch KF, Weitz CJ. 2008. Physiological significance of a peripheral tissue circadian clock. Proc Natl Acad Sci USA. epub ahead of print: www.pnas.org_cgi_doi_10.1073_pnas.0806717105 [DOI] [PMC free article] [PubMed]
- 24.La Fleur SE. 2003. Daily rhythms in glucose metabolism: suprachiasmatic nucleus output to peripheral tissue. J Neuroendocrinol 15:315–322 [DOI] [PubMed] [Google Scholar]
- 25.Matsuzawa T, Sakazume M. 1994. Effects of fasting on haematology and clinical chemistry values in the rat and dog. Comp Haematol Int 4:152–156 [Google Scholar]
- 26.Mistlberger RE. 1994. Circadian food-anticipatory activity. Formal models and physiological mechanisms. Neurosci Biobehav Rev 18:171–195 [DOI] [PubMed] [Google Scholar]
- 27.Mrosovsky N. 1988. Phase response curves for social entrainment. J Comp Physiol A 162:35–46 [DOI] [PubMed] [Google Scholar]
- 28.Nelson W, Tong JL, Lee JK, Halberg F. 1979. Methods for cosinor rhythmometry. Chronobiologia 6:305–323 [PubMed] [Google Scholar]
- 29.Piccione G, Caola G, Refinetti R. 2003. Circadian rhythms of body temperature and liver function in fed and food-deprived goats. Comp Biochem Physiol A Mol Integr Physiol 134:563–572 [DOI] [PubMed] [Google Scholar]
- 30.Piccione G, Caola G, Refinetti R. 2005. Daily rhythms of blood pressure, heart rate, and body temperature in fed and fasted male dogs. J Vet Med A Physiol Pathol Clin Med 52:377–381 [DOI] [PubMed] [Google Scholar]
- 31.Pittendrigh CS, Daan S. 1976. Functional analysis of circadian pacemakers in nocturnal rodents. 4. Entrainment—pacemaker as clock. J Comp Physiol A Neuroethol Sens Neural Behav Physiol 106:291–331 [Google Scholar]
- 32.Pittendrigh CS. 1993. Temporal organization: reflections of a Darwinian clock-watcher. Annu Rev Physiol 55:16–54 [DOI] [PubMed] [Google Scholar]
- 33.Refinetti R. 2004. Nonstationary time series and the robustness of circadian rhythms. J Theor Biol 227:571–581 [DOI] [PubMed] [Google Scholar]
- 34.Reppert SM, Weaver DR. 2002. Coordination of circadian timing in mammals. Nature 418:935–941 [DOI] [PubMed] [Google Scholar]
- 35.Rudic RD, McNamara P, Curtis AM, Boston RC, Panda S, Hogenesch JB, Fitzgerald GA. 2004. BMAL1 and CLOCK, two essential components of the circadian clock, are involved in glucose homeostasis. PLoS Biol 2:e377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stephan FK. 2002. The ‘other’ circadian system: food as a Zeitgeber. J Biol Rhythms 17:284–292 [DOI] [PubMed] [Google Scholar]
- 37.Stephan FK, Swann JM, Sisk CL. 1979. Anticipation of 24-h feeding schedules in rats with lesions of the suprachiasmatic nucleus. Behav Neural Biol 25:346–363 [DOI] [PubMed] [Google Scholar]
- 38.Stephan FK, Swann JM, Sisk CL. 1979. Entrainment of circadian rhythms by feeding schedules in rats with suprachiasmatic lesions. Behav Neural Biol 25:545–554 [DOI] [PubMed] [Google Scholar]
- 39.Turek FW, Joshu C, Kohsaka A, Lin E, Ivanova G, McDearmon E, Laposky A, Losee-Olson S, Easton A, Jensen DR, Eckel RH, Takahashi JS, Bass J. 2005. Obesity and metabolic syndrome in circadian Clock mutant mice. Science 308:1043–1045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Van Gelder RN. 2003. Making (a) sense of nonvisual ocular photoreception. Trends Neurosci 26:458–461 [DOI] [PubMed] [Google Scholar]
- 41.Wijnen H, Young MW. 2006. Interplay of circadian clocks and metabolic rhythms. Annu Rev Genet 40:409–448 [DOI] [PubMed] [Google Scholar]
- 42.Zanzinger J, Hoffmann I, Becker K. 1994. Diurnal variations in blood gases and metabolites for draught Zebu and Simmental oxen. Comp Biochem Physiol Comp Physiol 108:169–173 [DOI] [PubMed] [Google Scholar]
- 43.Zhang J, Kaasik K, Blackburn MR, Lee CC. 2006. Constant darkness is a circadian metabolic signal in mammals. Nature 439:340–343 [DOI] [PubMed] [Google Scholar]