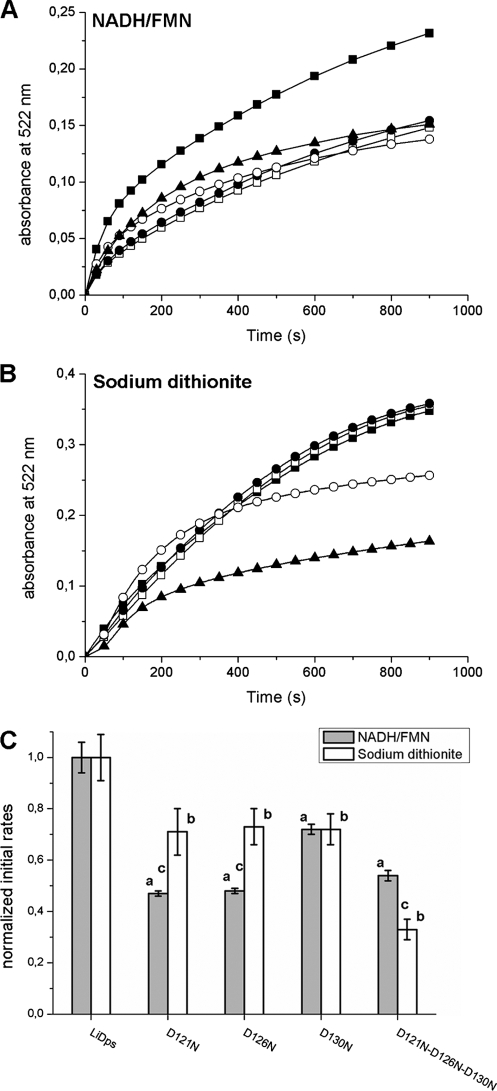
FIGURE 6.
Aspartate 121, 126, and 130 each contribute to iron removal from the hydrated ferric oxide mineral inside the L. innocua Dps protein. A and B, the iron release from LiDps and pore site-specific aspartate variants was monitored following the formation of the [Fe(II)-bipyridyl3] complex at 522 nm. Reduction and chelation were triggered by adding 2.5 mm of reductants (NADH/FMN (A) or sodium dithionite (B)) and 2.5 mm bipyridyl to solutions of protein with mineral (0.2 μm protein and 48.0 μm iron) in 100.0 mm MOPS, pH 7.0. ■, LiDps; □, D121N; ●, D126N; ○, D130N; ▲, D121N/D126N/D130N. C, initial rates of iron release calculated as described in Table 2. Each bar represents the value of the initial rate normalized for the LiDps value. The data are averages of five or six independent experiments, and the error is presented as standard deviation. a, D121N, D126N, D130N, and D121N/D126N/D130N variants are significantly different from LiDps (p < 0.0001) when iron release is triggered by NADH/FMN. b, D121N, D126N, D130N, and D121N/D126N/D130N variants are significantly different from LiDps (p ≤ 0.0002) when iron release is triggered by sodium dithionite. c, D121N, D126N, and D121N/D126N/D130N are significantly different (p ≤ 0.0002) when iron release is performed with NADH/FMN or sodium dithionite.