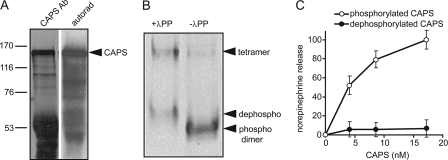
FIGURE 1.
Phosphorylation is essential for CAPS activity. A, CAPS was immunoprecipitated from extracts of 32P-labeled PC12 cells and analyzed on 7.5% SDS gels for Western blotting with CAPS antibody (Ab, left lane) or by autoradiography for 32P (right lane). B, recombinant CAPS purified from Sf9 cells (right lane) was incubated with λ-protein phosphatase (λPP, left lane) and analyzed by electrophoresis on native 6% polyacrylamide gels and Coomassie staining. Bands from the bottom to top correspond to native phosphorylated dimer (phospho), dephosphorylated dimer (dephospho), and higher molecular weight tetramer. C, native phosphorylated (○) but not dephosphorylated CAPS (●) restores Ca2+-triggered release of [3H]norepinephrine from permeable PC12 cells. Permeable cells were prepared as described under “Experimental Procedures” and incubated for 3 min at 30 °C with 10 μm Ca2+ plus the indicated concentrations of CAPS. Mean values of duplicate determinations with the indicated ranges correspond to the CAPS-dependent component of norepinephrine release with maximal value set at 100% for wild type CAPS.