FIGURE 2.
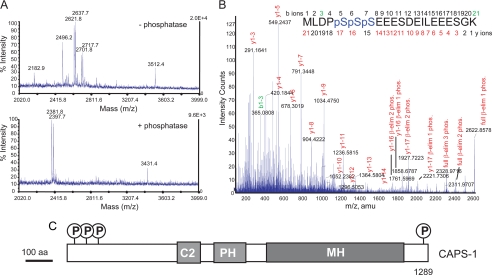
CAPS is phosphorylated at N- and C-terminal Ser residues. A, MALDI-TOF spectra of CAPS phosphopeptides. Phosphopeptides enriched from CAPS tryptic peptides by Ga(III)- immobilized metal ion affinity chromatography were incubated without (upper panel) or with (lower panel) alkaline phosphatase before MALDI-TOF to detect mass shifts of −80 Da (HPO3−) or multiples of −80 Da. B, MALDI-QTOF MS/MS spectrum of triply phosphorylated CAPS residues 1–21 (2622.9 atomic mass units (amu)) with a summary of Ser phosphorylations detected by neutral loss of H3PO4 by β-elimination (β-elim). C, schematic figure of CAPS indicating phosphorylation sites relative to other functional domains. Functional domains correspond to C2 (protein kinase C C2-like), PH, and MH (munc 13 homology).