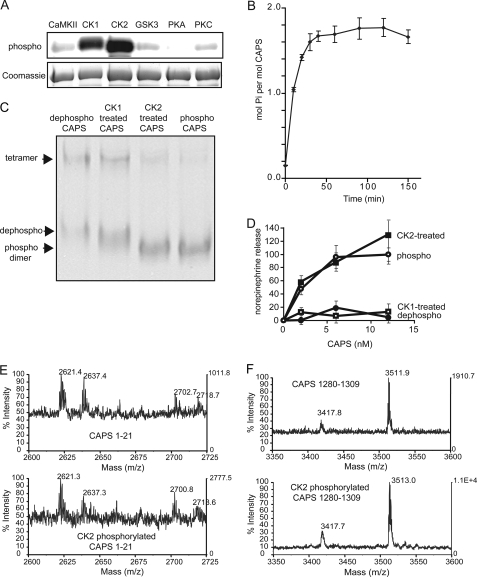
FIGURE 5.
CK2 phosphorylates CAPS in vitro and restores activity. A, in vitro phosphorylation of CAPS by Ca2+/calmodulin-dependent protein kinase II, CK1, CK2, glycogen synthase kinase 3, protein kinase A (PKA), and protein kinase C (PKC). Dephosphorylated CAPS was incubated with the indicated enzymes in the presence of [32P]ATP. Proteins separated by 7.5% SDS-PAGE were analyzed by phosphorimaging (upper panel) and Coomassie G250 staining (lower panel). B, the approximate stoichiometry of phosphorylation of 20 μg of dephospho-CAPS by 50 units of CK2 was determined at the indicated incubation times at 30 °C. C, CAPS proteins were analyzed by 6% acrylamide native gel electrophoresis and staining with Coomassie G250. Phosphorylated dimer, dephosphorylated dimer, and tetramer are indicated by arrowheads from bottom to top. D, activity of CAPS phosphorylated by CK1 or CK2. Dephosphorylated CAPS (●) incubated with either CK1 (□) or CK2 (■) plus MgATP were re-purified and tested for activity in Ca2+-dependent [3H]norepinephrine release from permeable PC12 cells. Maximal release promoted by native phosphorylated CAPS (○) was set as 100%, and release with Ca2+ alone was set as 0%. Values are the mean of duplicate determination with indicated ranges. E and F, native phosphorylated CAPS (upper panels) and dephosphorylated CAPS incubated with CK2 and MgATP (lower panels) were compared by MALDI-TOF after trypsin digestion. MALDI-TOF spectra corresponding to CAPS 1–21 (E) and CAPS 1280–1309 (F) are shown.