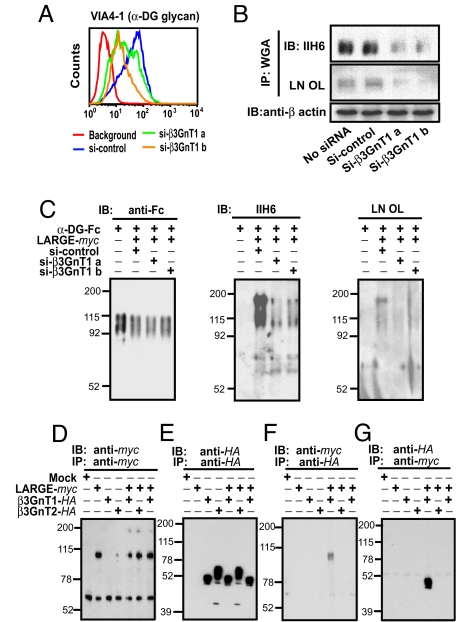
Fig. 3.
β3GnT1 facilitates functional glycosylation of α-DG by cooperating with LARGE. PC3-H cells were treated with siRNA to knock down β3GnT1, and the expression of laminin-binding glycans on α-DG was examined by FACS (A) and immunoblotting (B). (C) Effects of knockdown of endogenous β3GnT1 by siRNA on the laminin-binding glycan formation induced by LARGE overexpression in α-DG–Fc secreted from HEK293 cells. (D-G) β3GnT1-HA, LARGE-myc, and β3GnT2-HA were separately expressed or coexpressed in CHO cells and immunoprecipitated. Dissociated immunoprecipitates were separated, blotted, and incubated with specific anti-tag antibodies. LARGE-myc and β3GnT1-HA in far right lanes were prepared by mixing the lysates derived from cells separately expressing LARGE-myc or β3GnT1-HA.