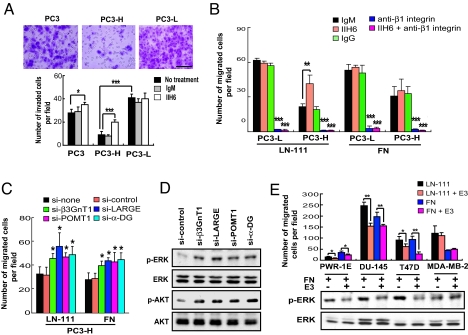
Fig. 4.
Laminin-binding glycans on α-DG attenuate cell migration and integrin-dependent signaling pathways. (A) Cell invasion assay with and without antibody treatment. The invaded cells were stained with crystal violet and counted. (Scale bar: 500 μm.) (B) Cell migration assay of PC3-L and PC3-H cells on laminin-111 (LN-111) and fibronectin (FN). Effects of pretreatment with IIH6, anti-β1 integrin, and anti-β1 integrin plus IIH6 on the cell migration are also shown. (C) Effects of siRNA-mediated knockdown of α-DG, POMT1, β3GnT1, or LARGE on the cell migration on LN-111 and FN. (D) ERK and AKT kinase activation levels in PC3-H cells treated with siRNA as indicated. Antibodies specific to phosphorylated ERK (p-ERK), phosphorylated AKT (p-AKT), total ERK (ERK), and total AKT (AKT) were used. (E) Effects of LN-111 E3 fragment on cell migration and ERK phosphorylation. (E, Upper) Cell migration was measured using a transwell. E3 alone did not induce cell migration. (E, Lower) Phosphorylation of ERK of various prostate and breast carcinoma cells treated for 20 min with FN alone or with FN plus E3 and analyzed by immunoblotting (see Fig. S6E).