Abstract
Background and Aims
Respiratory critical oxygen pressures (COPR) determined from O2-depletion rates in media bathing intact or excised roots are unreliable indicators of respiratory O2-dependency in O2-free media and wetlands. A mathematical model was used to help illustrate this, and more relevant polarographic methods for determining COPR in roots of intact plants are discussed.
Methods
Cortical [O2] near the root apex was monitored indirectly (pea seedlings) from radial oxygen losses (ROL) using sleeving Pt electrodes, or directly (maize) using microelectrodes; [O2] in the root was controlled by manipulating [O2] around the shoots. Mathematical modelling of radial diffusive and respiratory properties of roots used Michaelis–Menten enzyme kinetics.
Key Results
Respiration declined only when the O2 partial pressure (OPP) in the cortex of root tips fell below 0·5–4·5 kPa, values consistent with depressed respiration near the centre of the stele as confirmed by microelectrode measurements and mathematical modelling. Modelling predictions suggested that the OPP of a significant core at the centre of roots could be below the usual detection limits of O2-microelectrodes but still support some aerobic respiration.
Conclusions
In O2-free media, as in wetlands, the COPR for roots is likely to be quite low, dependent upon the respiratory demands, dimensions and diffusion characteristics of the stele/stelar meristem and the enzyme kinetics of cytochrome oxidase. Roots of non-wetland plants may not differ greatly in their COPRs from those of wetland species. There is a possibility that trace amounts of O2 may still be present in stelar ‘anaerobic’ cores where fermentation is induced at low cortical OPPs.
Key words: Critical oxygen pressure, cytochrome oxidase, maize, modelling, non-wetland, tissue O2 profiles, pea, radial oxygen loss, root aeration, root respiration, stelar O2 deficiency, wetland
INTRODUCTION
In root physiology the term ‘critical O2 pressure’ originates from studies of onion root respiration by Warburg manometry conducted by Berry (1949) and Berry and Norris (1949): O2 uptake by root segments bathed in phosphate buffer was determined over a range of O2 concentrations and temperatures. The plots of concentration vs. uptake proved to be consistent in pattern, irrespective of temperature, and resolved into two parts. In the initial phase, respiratory activity rose sharply with increasing O2 partial pressure (OPP), reaching a plateau, the second phase, when respiratory activity was at its maximum and independent of O2 pressure. The [O2] at the intersection of these two phases, the critical O2 pressure (hereafter COPR), is the lowest concentration to support maximum respiratory rate and below which the rate of O2 consumption rapidly declines. As will be discussed later, diffusive resistances play a major role in shaping the initial phase, while in phase 2 respiratory activity is independent of diffusion constraints.
Various reasons might prompt measurement of root COPR. One would be to learn whether COPR might be type-specific: could wetland plants have inherently lower COPRs than non-wetland plants, conferring competitive advantage in wet, anaerobic, soils. From our knowledge of the various methods used to measure COPR it is possible to identify a number of factors that can influence what is identified as the COPR (or ‘perceived’ COPR). These include: (1) the position at which O2 is monitored, (2) stirring rates if in a liquid medium, (3) morphological and anatomical characteristics of the roots, (4) oxygen demand, (5) whether the measurements are on excised/intact roots, and (6) the O2 affinity of the enzyme system(s). This paper begins with a brief overview of the theory and methodology of COP measurements, including an analysis of the effects that these factors can have on COPR values; where appropriate, to aid this analysis, a mathematical model describing radial O2 diffusion into roots and based on Michaelis–Menten kinetics has been used. Secondly, this paper reports on experiments to test the hypothesis that non-wetland plants differ little in COPR from wetland species. Intact plants of two non-wetland species, pea and maize, were used and, to mimic the normal wetland condition, the medium bathing the roots was kept anoxic so that the only respiratory O2 supplied was from the shoot by internal gas-phase diffusion. Internal OPP and hence respiratory activity in the roots was controlled by manipulating the oxygen regime around the shoot (OPPshoot). Internal OPPs close to the root apex (OPProot) were monitored either from localized measurements of radial oxygen loss (ROL) or from O2-microelectrodes probing the root cortex. COPRs were determined from plots of ROL or root OPP vs. OPPshoot.
MATERIALS AND METHODS
Mathematical modelling
To help assess the significance of COPRs obtained by methods involving the scavenging of O2 in respiratory cells and other containers, some use has been made of a mathematical diffusion-based multicylindrical model designed to predict the radial distribution of oxygen within roots receiving oxygen radially from the soil or bathing medium. The model is a development of that described by Armstrong and Beckett (1985) but with respiration rate and OPP at any locus modelled according to Michaelis–Menten kinetics rather than previously when respiration was treated as a zero-order reaction at any locus, unaffected by oxygen concentration until anoxia, at which point respiration would cease. The stele was modelled as three cylinders: an inner (medulla; r = 0·04 mm), middle (late metaxylem; r = 0·15 mm) and outer (protoxylem, early metaxylem, phloem, pericycle and endodermis; r = 0·23 mm); the cortex was given a radius of 0·36 mm; the epidermal/hypodermal cylinder a radius 0·44 mm and provision was made for a diffusive (non-respiring) boundary layer (DBL) around the root, the radius of which was set to a range of values. Beyond the boundary layer turbulent flow and here uniform O2 concentration was assumed. Respiratory activity, M (ng cm−3 s−1), and diffusivity, D (cm2 s−1), data programmed into the model were those necessary to obtain predictions close to the experimental result shown later in Fig. 5. These were as follows: medulla (M, 150; D, 1 × 10−5, i.e. 4687 µmol m−3 s−1 and 1 × 10−9 m2 s−1, respectively); metaxylem (M, 25; D, 1 × 10−5); phloem/pericycle (M, 250; D, 1 × 0−5); cortex (M, 25; D, 2 × 10−3); epidermis/hypodermis (M, 150; D, 2 × 10−5). The respiratory data used were within the limits of measurements previously made on maize roots (Armstrong et al., 1991).
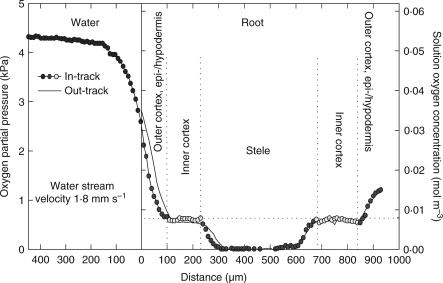
Fig. 5.
In-track (symbols) and out-track (continuous line) microelectrode radial O2 profiles across an excised primary root of maize lying in a nutrient medium that was streamed lengthwise past it at a velocity of 1·8 mm s−1. The dissolved O2 concentration in the medium was 0·054 mol m−3 (4·3 kPa); the profile was at 75 mm from the apex and total root length was 135 mm. Temperature, 25 °C. (Modified from Gibbs et al., 1998, where the depth of the vessel was wrongly given as 100 mm instead of 10 mm and the flow velocity given was incorrect.)
COPR determinations
Principle
When the roots of intact plants are submerged in an anoxic/hypoxic medium and the shoot emergent, oxygen diffuses from the aerial parts to accommodate root respiration, and some is lost from the roots into the bathing medium. The internal longitudinal flow of O2 to the apical region and the ROL from it to an ensheathing cylindrical polarographic Pt electrode have been likened to the flow of electricity in a simple circuit (Armstrong & Wright, 1975). The difference in OPP between the atmosphere around the shoot and the electrode surface may be represented as the concentration difference, ΔC (equivalent to a voltage difference, V), the O2-diffusion rate from root to electrode as J (equivalent to electrical current flow, I), the non-metabolic (pore-space) resistance of the root as Rp, and the diffusive resistance through the liquid shell between root and electrode as Rs. If the respiratory O2 consumption in the root is represented as a component, RESP, then, following Ohm's law (I = V/R), a formula that describes the O2-diffusion from root to electrode may be written as:
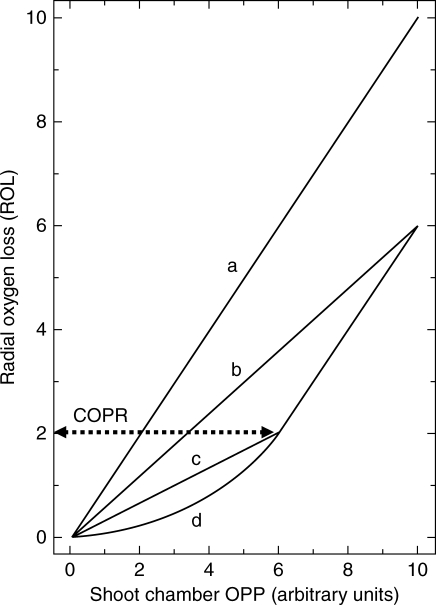
where J represents the ROL from root to electrode in g s−1, ΔC has units of g cm−3 and resistances have units of s cm−3. Based on this equation, Armstrong & Gaynard (1976) made the following predictions concerning the relationship between ROL and varying OPPshoot. If, for any given root, Rp + Rs is constant, and this is very likely over short experimental periods, then I could increase with V in a number of ways (see Fig. 1): with zero respiration I should increase linearly with V (Fig. 1, plot a). If respiration is significant and increases linearly with V, the relationship between I and V again will be linear, but divergent from and lying below plot a (Fig. 1, plot b). If an initial linear increase is followed by a constant level of respiration above some critical value of V there will be an inflexion at this point (indicative of the COPR) and the line should turn and run parallel to the non-respiratory plot a (Fig. 1, plot c). Alternatively, if, below the COPR, respiration is a curvilinear function of V, the initial section of the plot below the COPR will be curvilinear (Fig. 1, plot d). The inflexion point indicates the COPR, and the ROL at this point translates into a root surface O2 concentration from the expression: ROL = Csurface/Rs (Armstrong and Wright, 1975). The cortical OPP at the COPR will be greater than the root surface OPP by an amount equal to the sum of the oxygen deficits across the gas–space–free epidermal/hypodermal layers (root wall). These are created by respiration within the wall layers and by O2 leakage through the wall to the electrode. An equation that can be used to estimate these values can be found in Armstrong and Webb (1985). This requires a measurement of the thickness of the gas–space–free root wall (from root transverse sections) and some knowledge/qualified estimate of respiratory demand and O2 diffusivity in these layers. Near to the apex of an actively growing root the O2 diffusivity of the epidermis/hypodermis tends to be very high (close to that for O2 in H2O; see also Garthwaite et al., 2008).
Fig. 1.
Predicted relationships between apical radial O2 loss (ROL) and shoot chamber O2 concentration (OPPshoot) when respiration is (a) zero, (b) a linear function of OPPshoot, (c) initially a linear function of OPPshoot but becoming constant at the critical O2 pressure (COPR) and (d) initially a curvilinear function of OPPshoot but becoming constant at the COPR. Arbitrary units used for shoot chamber OPP.
In practice, the x-intercepts for plots of ROL vs. OPP around the shoot are invariably right-shifted, particularly for a non-wetland species such as pea. This reflects (1) the O2 consumption across the epidermal/hypodermal cell layers – when cortical OPPs are low this removes all radially diffusing O2, and so prevents ROL from the root, and (2) the shoot to root O2-concentration gradient.
Based on the above principles the COPR for pea roots was determined using ROL measurements (indirect method) while for maize roots, cortical OPP was measured directly using O2-microelectrodes (direct method). The pea experiments were performed before O2-microelectrodes became available.
Indirect method
ROLs were determined polarographically: for additional detail see Armstrong and Wright (1975) and Armstrong (1979).
Seeds of Pisum sativum L. ‘Meteor’ were surface sterilized (3 min in 0·1 % mercuric chloride solution) rinsed several times and soaked for 24 h in sterile distilled water. They were then germinated over 1–2 d at 25 °C wedged upright against damp tissue in a sterile crystallising dish. When the radicles were 1–2 cm long, the seeds were transferred aseptically to 250-cm3 Pyrex cylinders containing approx. 180 cm3 of sterilized 1 % agar in quarter-strength Hoagland's solution. The radicle was fitted into a hole in the agar and the seed rested on the agar surface. Each cylinder possessed a glass release-rod and basal extraction disc and ties to allow the subsequent removal of the agar and seedling. A black polythene wrap was used to exclude light from the agar and seedlings were allowed to develop in a growth room at 23 °C; a photosynthetic light flux of 100 µmol m−2 s−1 was provided by cool fluorescent lights with a daylength of 16 h.
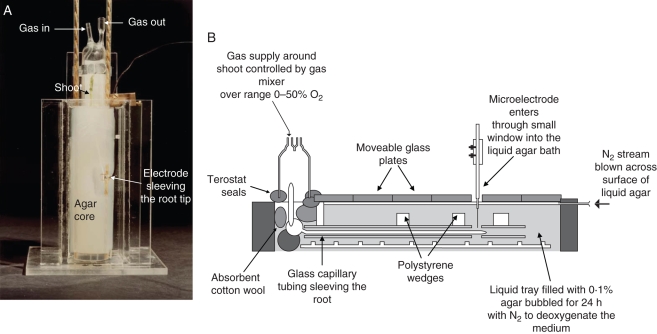
When the primary root was between 70 and 100 mm long, agar and plant were extracted en bloc, any laterals were excised in situ and the whole transferred to a close-fitting glass tube. A cut-away section in the tube made it possible to trim away sufficient agar to expose the apical 1·5 cm of primary root. Agar-core and tube were then immersed in close to O2-free 0·05 % agar–water medium (plus supporting electrolyte: 7 mm KCl) in a Perspex vessel (Fig. 2A) and the liquid surface raised level with the root–shoot junction. As the stomata immediately above the surface form the effective entry point for O2 transport to the root, the shoot makes a negligible contribution to the resistance path (Armstrong, 1979). For measuring ROL a cylindrical platinum electrode (height 5 mm, inner diameter 2·25 mm) was positioned and maintained, sleeving the root 2–5 mm from the tip, the meristem and root cap just protruding from its lower end. Root radius at the electrode position ranged from 0·4 and 0·63 mm [mean 0·48 ± 0·02 mm (s.e.)]; gas–space–free hypodermal/epidermal cell cylinder thickness ranged from 27 to 35 µm. Light was excluded from the root chamber by placing black card against its vertical sides. Shoot and cotyledons were enclosed in a glass hood containing moistened filter paper to prevent shoot desiccation and the OPP within the root apex was controlled by supplying humidified and pre-calibrated commercial O2/N2 gas mixtures to the hood via an inlet tube opening at the level of the seed; from the top a tube vented through a small head of water in an adjacent vessel for observing and controlling the flow rate. In experiments on wetland plants (Armstrong and Gaynard, 1976) air was the first gas-mixture applied around the shoot to ensure that the OPP in the root apex was initially high; the shoot OPP was then sequentially lowered. To achieve similar internal OPPs in pea roots the initial gas-mixture had an OPP of approx. 50 kPa; this was necessary because of the steep O2 concentration gradient from shoot to root arising from the greater effective diffusive resistance to gaseous O2 transport in the pea root compared with wetland species (fractional root porosity of pea roots, even in agar, does not exceed 0·038: Armstrong et al., 1983). The OPP around shoot was then lowered to approx. 15 kPa in eight or nine stages and the ROL, when at equilibrium, recorded at each stage. A current–voltage curve was taken also at each stage to establish the correct and appropriate plateau potential [(–) EMFPt vs. saturated Ag:AgCl reference electrode] for electrolytic O2 reduction: this is critical because of the drift to less negative potentials at lower OPPs when using large naked cathodes (Armstrong, 1979). In some experiments the first OPP used was a low one, with subsequent stepwise changes to higher OPPs. There were no obvious differences in the results obtained by the two approaches.
Fig. 2.
(A) Pea seedling with root system, except the apical region, held in a solid agar jacket and arranged in anaerobic 0·05 % agar/water medium with root apex ensheathed by a cylindrical Pt electrode. Shoot enclosed in humidified glass hood with gas-ports to enable changes to OPPshoot. (B) Assembly for effecting changes to OPPshoot for maize root COPR measurement by O2-microlectrodes.
As the shape of pea roots changes with increasing length (Armstrong et al., 1983) and as ROL equilibration periods for non-wetland plants can be quite long, the root radii at the top and bottom of the electrode position were measured at each gas-mixture stage, as was the length of root below the electrode. This was carried out in situ using a vernier-equipped travelling microscope with a protractor graticule in the eyepiece. The mean of these two radii was used to calculate the surface area of root within the electrode at each gas-mixture stage. All experiments were carried out at approximately 23 °C. Finally, for each plant tested, equilibrium values of ROL were plotted against the matching shoot OPP; in total 19 plants were tested.
Direct method
Maize seedlings were surface sterilized and germinated as described for pea. Seedlings with short roots were then transferred to sterile, narrow, agar-filled tubes (internal diameter 10 mm) embedded in 1 % agar-filled cylinders and grown under sterile conditions at 17 °C. The narrowness of the tubes helped in obtaining a high proportion of straight roots. When the roots were an appropriate length (approx. 85–105 mm) the seedlings were transferred to the apparatus shown in Fig. 2B and all experiments were conducted at a room temperature of approximately 21 °C. At this stage, although the apical parts were non-aerenchymatous with fractional porosities of ≤0·07, aerenchyma developed sub-apically and fractional porosities could reach as high as 0·30 (Darwent et al., 2003). The roots were threaded into close-fitting glass tubes and secured horizontally in a long rectangular trough containing nutrient 0·1 % agar–water medium that had been autoclaved and bubbled with O2-free N2 for at least 24 h; the shoots were enclosed in a hood similar to that described for pea. The ‘roof’ of the trough was covered in moveable glass plates and the points of contact between trough wall and the plates were covered with Si-grease to help prevent room air entering the 5-mm-deep head space between the plates and the rooting medium through which a stream of N2 gas was gently and continuously blown. The plates were moved apart very slightly (5 mm) at one point only to allow the vertical entry of an O2-microelectrode into the chamber to probe the root. The OPP within the chamber enclosing the seed, the shoot and coleoptile sheath was supplied with compressed air (21 kPa O2) or with different OPPs using a gas-blender (Signal: series 850). A bleed-link diverted some of the supply into a separate cell containing a Clark-type electrode. This electrode, connected to a polarograph (Barman Electronics, Skipsea, East Yorkshire, UK) was used to monitor the OPP in the mixtures supplied to the shoot and the gas-blender was manually controlled to provide the appropriate OPPs.
OPPs in the cortex or stele at approx. 5 mm from the root tip were measured polarographically using Clark-type gold-tipped O2-microelectrodes (tip diameters 5–12 µm; Armstrong et al., 2000); these were step-driven into the root by a computer-controlled servo-system. Most measurements were carried out with the electrode tip at a fixed point in the root cortex, but some radial O2 profiles were recorded, and some manipulations of shoot OPP performed, with the electrode tip positioned in the stele. The polarograph also was computer-controlled and OPPs were recorded ‘continuously’ using convenient time intervals (1 s, 10 s, 1 min, etc.) and stored digitally. For each experiment the shoot OPP was first set to a high value, e.g. 50 kPa, or a low value, e.g. 10 kPa, and, when the root OPP had reached equilibrium, the OPP around the shoot was subsequently reduced, or increased, in stages, and the root OPP was allowed to equilibrate at each stage. Over the lifetime of each multiple gas-change experiment, often 4–8 h, up to 20 such changes could be made. The time taken to reach equilibrium varied and depended largely upon the nature of the axial transport path and the magnitude of the gas-change. For example, changes which lowered the root OPP to below the COPR often produced an oscillation and a ‘lag’ period which could double equilibration times. Similarly, changes which reduced or increased root OPP at OPPs already below the COPR could produce such oscillations. We are uncertain as to their cause but they are suggestive of some delayed stelar respiratory responses to decreasing or increasing cortical OPPs.
Finally, for each of the seven plants tested, equilibrium internal root OPP values were plotted against the matching shoot OPP.
Respiration vs. O2 concentration – excised roots
Freshly germinated peas (‘Meteor’) were planted in vermiculite to a depth of approx. 20 mm in 120-mm-diameter plastic pots and harvested within 7 d when the roots were approx. 120 mm long and the plumule just emergent. The relationship between respiratory activity and O2-concentration in the bathing medium was determined on excised root segments using a constant-temperature water-jacketed Rank (Cambridge, UK) polarographic (Clark-type) respirometer assembly plus magnetic stirrer and chart recorder. The apical 40 mm of 120-mm-long roots were cut into batches of 30 4-mm-long segments (i.e. three roots per batch) and transferred to 3 ml of 3 % air-saturated glucose solution in the respirometer cell. The O2-depletion dynamics were monitored after re-inserting the respirometer lid and the respiratory rate at a range of O2-concentrations was determined from the slopes of the depletion curves. The measurements were made at 22·5 °C.
RESULTS AND DISCUSSION
Analysis of factors affecting COPR determinations
Enzyme O2-affinities, oxygen demand, diffusive resistance
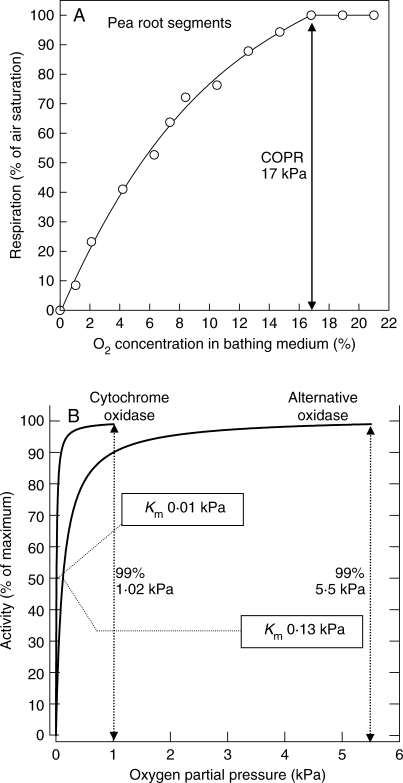
Derived from an O2-scavenging time-curve, Fig. 3A shows a typical plot of O2-uptake versus substrate OPP for pea root segments bathed in a stirred glucose-enriched medium. Similar in form to the original results for onion root respiration of Berry and Norris (1949), a relatively high COPR (17 kPa) is clearly indicated. Two of the major plant terminal oxidases, cytochrome oxidase and the alternative oxidase, have very different affinities for oxygen. For cytochrome oxidase the oxygen substrate concentration (Km) for half maximum uptake rate, approx. 0·14 µm (0·01 kPa O2) is an order of magnitude less than of the alternative oxidase, namely approx. 1·7 µm (0·13 kPa O2) and plots of their activities over the first phase of their respective COPR curves (Fig. 3B) reveal them to be almost fully O2-saturated (99 %) at approx. 1·02 and 5·5 kPa. Why then do the pea roots show a much higher COPR? Is it because of an oxidase with a very much higher Km than even the alternative oxidase or is there some other explanation? At the cellular level, e.g. with micro-organisms, where radial diffusive resistances will normally be very low, Km values are usually very low, namely 0·002–0·06 kPa and dependent upon cell size and shape as well as upon the properties of the terminal oxidase (Longmuir, 1954).
Fig. 3.
(A) The relationship between respiratory activity of pea root segments and O2 concentration in the bathing medium of a Rank (Clark-type) polarographic respirometer assembly with magnetic stirrer. The measurements were made at 22·5 °C using 30 4-mm-long segments from the apical 4 cm of three 12-cm-long roots bathed in 3 ml of 3 % glucose solution. (B) Michaelis–Menten curves derived by mathematical modelling and based on published Km values (Longmuir, 1954) for cytochrome oxidase and the alternative oxidase at the cellular level.
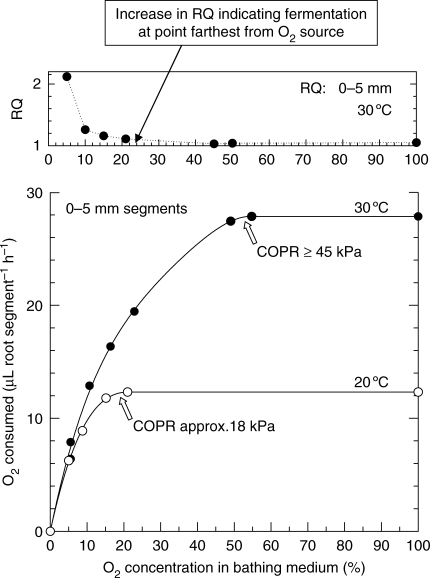
For onion root segments at 20 °C, Berry and Norris recorded COPRs of 21 (Fig. 4), 15 and 10 kPa O2, respectively, for the zones 0–5, 5–10 and 10–15 mm above the onion root apex. At 30 °C the critical pressures were approx. 45 (Fig. 4), 21 and 10 kPa, respectively. Oxygen consumption in the apical 5 mm of the onion root was twice that of the 5–10-mm zone and this latter zone respired at a rate greater than that at 10–15 mm from the apex (Berry and Brock, 1946). Consequently, the magnitude of the COPR was revealed to be dependent upon the respiratory rate of the tissue and on the temperature of the system. The discrepancy between high in vitro COP and the affinity of the cytochromes for O2 was attributed by Berry and Norris to the diffusional impedances within the tissues, creating a core of anaerobiosis at O2 concentrations below the COPR. Indeed, CO2 output below the COPR values was such that RQ values consistently exceeded unity, indicating a significant leve1 of fermentation (Fig. 4). High ADH and PDC activity in the stele of maize but not in the cortex at low cortical OPPs (Thomson and Greenway, 1991) also supports Berry and Norris's supposition of a developing core of anaerobiosis below the COPR. Non-porous tissues such as the stele and epidermal/hypodermal cell layers would normally be the most significant resistances to the radial transfer of O2, and the porous cortex the least significant. Consequently, the larger the stelar diameter and/or the thicker the non-porous epidermal/hypodermal cell layers, the higher might be the expected COPRs when assessed in this way. However, should the cortex become flooded, because of damage due to segmenting of the roots, and to their submergence and possible contraction of gas-filled intercellular space because of escaping bubbles or CO2 solubilization, this might also add significantly to the resistance.
Fig. 4.
Oxygen consumption (μL root segment h−1) and corresponding RQ values (CO2 evolved: O2 consumed) for 0–5-mm root tips of onion in relation to O2 concentration in the bathing medium and at two temperatures. Measurements by Warburg respirometry using 2-d-old roots. Drawn from Berry and Norris (1949).
The effects of bathing media, segment size, ageing and oxidase activity have also been explored in some detail in other plant tissues, e.g. for potato tubers (Thiman et al., 1954). They demonstrated very clearly the effects of tissue thickness and further that tissue ageing is a factor that must not be overlooked. Their results confirm how massive can be the apparent COPR when based on OPP monitoring in the bathing solution. Previous workers had found that respiration of potato slices was lowered when the partial pressure of oxygen is below that in air (Levy et al., 1948) a similar result to that of recent work by Geigenberger et al. (2000). However, Thimann et al. found that for freshly cut tissue discs of potato 0·5 mm thick (10 mm diameter) in a moist atmosphere at 15 °C, the plots of oxygen uptake versus OPP were indistinguishable from data for yeast cells (Longmuir, 1954): the Km was <0·1 kPa, and the COPR approx. 1 kPa; however, after 24 h ageing at 25 °C and measured at 15 °C, the COPR had risen to approx. 15 kPa and measured at 25 °C was approx. 20 kPa. It is difficult to reconcile these differences. The respiratory measurements of Geigenberger et al. were made on fresh (non-aged) material (slices of 1 mm thickness and 8 mm diameter) but in contrast to the measurements of Thimann et al., they were bathed in solution in an O2-electrode assembly. Consequently, it is conceivable that DBLs around the slices, together with some gas-space flooding, could have contributed to the 21 and 53 % decline in respiration found at, respectively, 8 and 4 % O2 in the bathing solution compared with that at air saturation (see below). Thus, they may also have contributed to corresponding changes in the tissue levels of adenine and uredine nucleotides and numerous other metabolites in tissue slices incubated at these oxygen concentrations. However, nucleotide and metabolite levels measured on slices plunged immediately into liquid nitrogen and taken from freshly extracted tuber cores were broadly in agreement with O2 concentrations found by O2-microelectrode measurements across the intact tubers and the corresponding values from the incubation experiments. Geigenberger et al. concluded that ‘low O2 triggers (i) a partial inhibition of respiration leading to a decrease of cellular energy status and (ii) a parallel inhibition of a wide range of energy-consuming processes’, the latter at O2 concentrations more than three orders of magnitude above the Km (O2) of cytochrome oxidase. Nevertheless, because of the difficulties in establishing the OPP levels in submerged materials (e.g. potato slices or root segments) that are being moved about in an incubation cell and hence surrounded by DBLs of indefinable dimensions, what still remains unclear is at precisely what OPP these changes might take place. It is conceivable that tissues taken at different distances from the centre of a tuber might have inherently different metabolisms or that there may be steep O2 gradients within the starch-packed cells themselves.
DBL effects and COPR in relation to sampling point
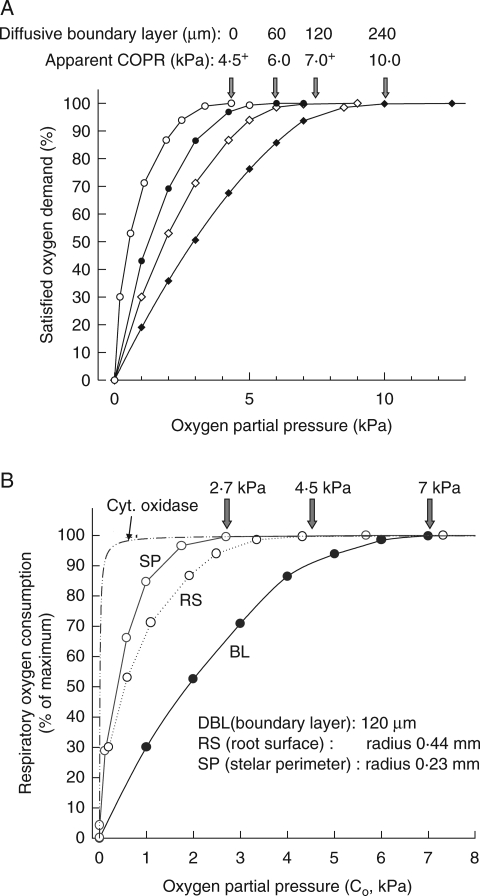
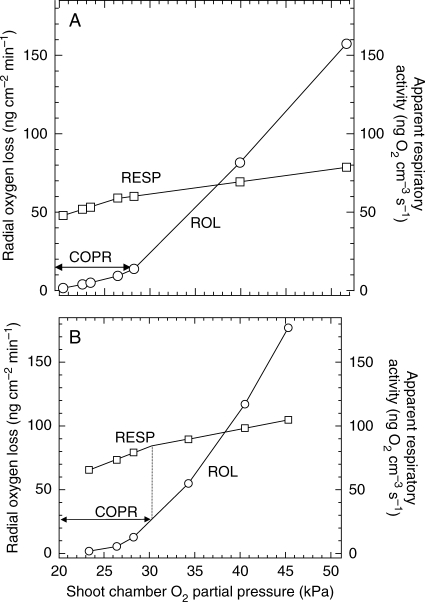
Another resistance markedly affecting the expression of COPR when oxygen is supplied radially from outside is the external DBL around any submerged root or root segment. O2-diffusion gradients across this layer and into and across the various root tissue cylinders are an expression of (1) the failure of O2 to return along the diffusion path, (2) path resistance and (3) consumption. The boundary layer, while not necessarily contributing to consumption, nevertheless slows the rate of supply into the root. In the root itself the various cell layers affect the rate of supply to tissues further in both because of their own resistance but also because of their removal of O2 (Armstrong and Drew, 2002). An appreciable DBL effect on radial diffusion of O2 into an excised maize root can be seen in Fig. 5. With 4·3 kPa O2 in a bathing medium streaming past the root at 1·8 mm s−1 the effective DBL extends radially to approx. 200 µm from the root surface and contributes 1·5 kPa to the oxygen deficit along the diffusion path. This root may already be below the COPR as the ‘anaerobic’ centre forecast by Berry and Norris appears to have developed. If the DBL could be significantly removed we should expect a drop in the COP recorded. Some results by Saglio et al. (1984) using maize root tips claimed to have shown this. Oxygen consumption versus OPP was measured with tips either (1) bathed in liquid and agitated or (2) exposed to moist air; both batches of roots were first vacuum-infiltrated with water in an attempt to minimize any differences that might have been attributable to cortical flooding. In moist air the COPR was only 10 kPa, but with a bathing medium it was 35 kPa and the DBL appeared to have been responsible for 25 of the perceived 35 kPa COPR. However, 0·5-cm-ong root tips were used in the bathing medium and on a per unit volume basis had approximately double the respiratory demand of the 1-cm-long tips used in the moist air. This considerable difference in demand is certain to have masked the true effect of any DBL. It is not absolutely clear how effective is vacuum infiltration on low-porosity blind-ended units such as root tips: Saglio et al. (1984) claimed that flooding of intercellular spaces had little effect on observed COPRs. However, in another of their data sets in moist air, infiltrated 1-cm root tips had a COPR of approx. 16 kPa compared with the COPR of 10 kPa for non-infiltrated roots: flooding of the spaces had apparently raised the COPR by approx. 6 kPa. In the mathematical simulations below and with a 120-μm DBL already in place, a flooding of the cortical gas-space raises the apparent COPR from just over 7 kPa to approx. 9·5 kPa; without a DBL it rises from approx. 4·5 to 7 kPa (profiles not shown).
The potential effects of boundary layers are perhaps best illustrated using mathematical simulations: Fig. 6A shows how DBL thickness would influence apparent COPR for a maize root having the diffusive and respiratory characteristics similar to the experimental example in Fig. 5, and with cytochrome oxidase as the major terminal oxidase. With no DBL at all, the COPR is close to 4·5 kPa; with a 60-μm DBL this rises to 6 kPa, 120 µm to just over 7 kPa and 240 µm it is approx. 10 kPa. The final value has more than doubled the apparent COPR. Physiologically, the only fixed point COPR is the 4·5-kPa surface concentration. This will remain the same whatever the DBL thickness, provided the OPP in the bathing medium is sufficient to satisfy fully the respiratory demand in the root.
Fig. 6.
(A) Mathematical modelling predictions of root respiratory activity based on maize root characteristics and showing the effect on apparent COPR (measured in the bathing medium) of different diffusive boundary layer thicknesses (DBL) from zero to 240 µm. (B) Mathematical modelling predictions of root respiratory activity based on maize root characteristics and showing how the perceived COPR varies with point at which OPP is measured; the DBL of 120 µm and the OPP of 7 kPa in the bathing medium was just sufficient to begin to reduce respiration at the root centre.
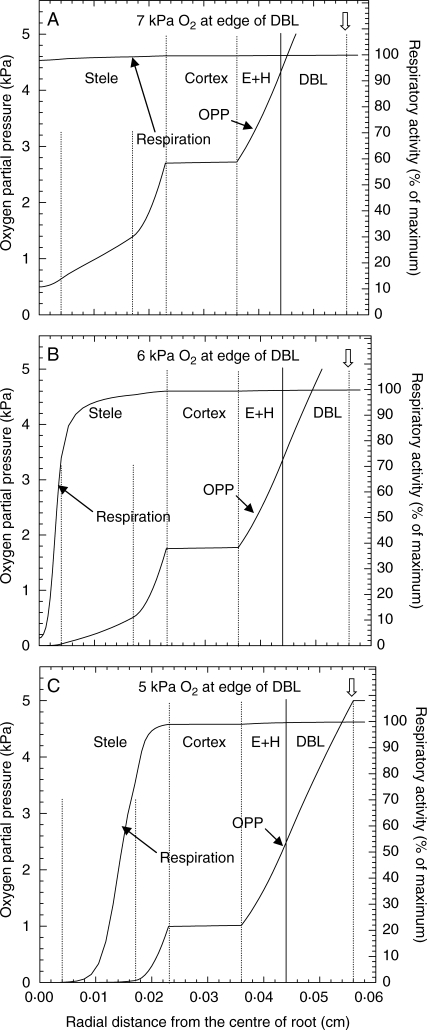
Using these mathematical simulations COPR can also be examined in terms of sampling points both outside and inside the root (see Fig. 6B) and in relation to respiratory activity inside the root (Fig. 7A–C). For a DBL thickness of 120 µm, and an oxygen partial pressure of 7 kPa in bulk medium, only just below the COPR and just beginning to lower the concentration at some point in the root below the saturation point of cytochrome oxidase, we can see that the concentration at the root surface would be approx. 4·5 kPa (our fixed point COPR in the previous example), while the COPR at the stelar perimeter is only 2·7 kPa (Figs 6B and 7A) and another approximate fixed point COPR. Where cytochrome oxidase is first noticeably affected (i.e. nearer to the root centre) the O2 concentration would of course be only approx. 1–2 kPa (Fig. 3B). If the OPP at the edge of the DBL is reduced to 6 kPa, stelar respiration becomes very noticeably reduced, and at the very centre is <2 % of maximum (Fig. 7B). With 5 kPa at the edge of the DBL something approaching an anaerobic core can be seen to have developed in the stele (Fig. 7C). A similar situation would develop with no DBL, a root surface OPP at 4·5 kPa and the cortical gas-spaces filled with water (data not shown). Although the plots in Fig. 7C appear to show that respiration has ceased in the inner cylinder of the stele and that here (and in Fig. 5) these tissues have become anoxic, this is not the case (at least in the modelling prediction). Rather, the OPP is well below the usual detection limits of O2-microelectrodes (0·007 kPa) and the respiratory activity is insufficient to show on the scales used in the figure. If the y-scales are expanded, O2 and respiration are still positive at radial distances that appeared to be anoxic in Fig. 7C and where fermentation has been shown to take place Thomson and Greenway (1991).
Fig. 7.
Modelling predictions of the radial oxygen profile and levels of respiratory activity across a maize root, with partial pressures of (A) 7 kPa, (B) 6 kPa and (C) 5 kPa, at the edge of the diffusive boundary layer (DBL). The predictions were based on simulations where the only oxygen source was in stirred solution culture bathing the root. The model assumed a Michaelis–Menten relationship for the oxygen dependency of respiration using a Km of 0·0108 kPa and the respiratory and diffusive characteristics of the various tissue cylinders as shown in the Methods section.
By using a Michaelis–Menten response curve for respiration vs. OPP, the mathematical modelling predicts that total anoxia is never reached but whether this applies in practice remains to be discovered, and what is meant by anoxia/anaerobic in this context needs to be defined or qualified. A consequence of using the Michaelis–Menten response curve for respiration vs. OPP (with its inherent assumption of continuity) is that there comes a point, when dealing with trace amounts of O2, when the results need to be treated with caution. What this point might be we are not yet certain, but we are confident that the model still functions satisfactorily in predicting to 10−5 kPa O2 and 0·1 % of maximum potential respiration.
It is relevant to note that if the Michaelis–Menten model for respiration is applied in a time-dependent system that has no diffusive inflow after time t = 0, when the concentration is uniform and has the value C0, then the concentration, C(t), at any subsequent time obeys the differential equation: dC/dt = –QC /(Km + C) and hence satisfies the implicit relationship:
From this we can deduce that for any value of time, C(t) will be positive, and hence we should never expect simple models based only on the Michaelis–Menten relationship to predict total anoxia.
If the mathematical predictions are true, and in view of the data of Berry and Norris (1949) and of Thomson and Greenway (1991), we are prompted to suggest that anaerobic metabolism at the centre of roots may proceed in the presence of trace amounts of free oxygen. It is tempting to speculate that some aerobic and anaerobic metabolism might occur spatially separated even in the same cell. It is well known that fermentation reactions occur in yeasts while there is still O2 present (Gonzhlez Siso et al., 1996; Otterstedt et al., 2004).
Intact versus excised roots
More reliable experimental results might be anticipated from determinations of COPR on non-segmented roots as this would certainly preclude flooding of the intercellular gas-space. However, when sampling the OPP in the bathing medium, it is important to appreciate that any COPR detected will depend not only upon DBL thickness (and stirring rate) but also upon whether the roots have been excised or kept intact. Using intact mustard seedlings, with the shoots in air and the roots bathed in a fast moving nutrient solution (≤25 cm s−1; DBL ≤4 µm) in a closed circuit system at 25 °C, Greenwood and Goodman (1971) found that the rate of O2-depletion from the nutrient solution first slowed at an OPP (and apparent COPR) of 12 kPa, and net O2 uptake had almost ceased by the time respiratory scavenging had reduced the solution OPP to approx. 5 kPa. This indicated not that root respiration had ceased, but that an appreciable part of the respiratory demand of the roots was being met by O2 from the shoot. When roots were excised and the cut ends sealed, the rate of O2 uptake by whole intact mustard roots did not fall significantly until O2 partial pressures were at 2 (Greenwood, 1968) to ≤5 kPa (Greenwood and Goodman, 1971) and substantially lower than the maize root COPRs discussed in the previous section. Several reasons could account for this: (1) the fast stirring rate in the mustard root experiments, (2) a lower O2-demand and (3) narrower roots than maize offering less radial diffusive resistance, particularly in the epidermal/hypodermal layers and the stele.
The examples so far demonstrate the difficulties inherent in establishing meaningful values of root COPR where measurements are based on sampling the OPP in the bathing medium from which roots are scavenging the oxygen. There is no obvious evidence to suppose that the high COPRs that have been found in this way are pointers to any substantial involvement of terminal oxidases other than cytochrome oxidase. The data confirm the conclusions of Berry and Norris (1949) that the COPR is a function of respiratory decline at the root centre; they also show that the values obtained can be as much a function of DBL resistance as internal diffusive impedance, and that gas-space flooding could also significantly distort any findings. However, even if DBLs could be eliminated or defined precisely, COPRs determined as above can be meaningful only for helping to establish conditions necessary for providing roots with sufficient O2 in hydroponics or in non-waterlogged soils. They can reveal little of value with regard to root COPRs in the wetland situation where there is little or no O2 in the medium around the root. In these circumstances it must be supplied by gas-phase diffusion from the shoot or rhizome.
It is well established that wetland plants are usually better able to obtain O2 in this way than non-wetland species because of their ability to enhance cortical gas-space provision and reduce respiratory demand by aerenchyma formation (Armstrong, 1979; Justin and Armstrong, 1987; Colmer, 2003; McDonald et al., 2002). In this way they are also better able to maintain micro-aerobic conditions in the rhizosphere by ROL (Justin and Armstrong, 1987; Sorrell et al., 2000; Jensen et al., 2005). Within the root itself it is the gas-space continuum of the cortex that is the immediate source of respiratory O2 not only for the cortical cells but for the non-porous stelar tissues (phloem, xylem and their parenchymas, and usually the pith, if present) and for the hypodermal/epidermal cell layers (Armstrong and Beckett, 1987; Sorrell et al., 2000). Aerobes and chemical oxidations in the rhizosphere are also dependent upon O2-diffusion from the cortex (Begg et al., 1994; Gilbert and Frenzel, 1998; van Bodegom et al., 2001; Kirk and Kronzucker, 2005). It follows that, to define the respiratory tolerance of a root in these circumstances, the sampling point for COPR determination should be the cortex. The values obtained should be significantly less than in the previous examples discussed as they do not involve any inward transport through a DBL and epidermal/hypodermal non-porous respiring tissues. Consequently, if cytochrome oxidase is the major terminal oxidase there might be no reason to suppose that COPRs will be substantially different between wetland and non-wetland species. Root COPRs in intact rice and Eriophorum angustifolium (cotton grass) determined from plots of ROL against leaf O2 concentration (Armstrong and Gaynard, 1976) were 2·4 and 2 kPa, respectively, an order of magnitude less than values recorded by Berry and Norris (1949), Luxmoore et al. (1970) and Scotter et al. (1967) and are probably a much truer reflection of the in vivo relationship between apical O2 concentration and root respiratory activity. They also suggest a dominant role for cytochrome oxidase: oxidases with a higher Km than cytochrome oxidase (e.g. see Fig. 3B) should result in significantly higher COPRs. In the modelling examples such as Fig. 7 where DBL significantly affected the perceived COPR, this was just in excess of 7 kPa; if respiratory demand had been dependent on the alternative oxidase, Km (O2) 0·13 kPa, it would have been >10 kPa. Thus far, data on COPR in mesophytes are limited to those based upon O2 uptake from the bathing medium as described above; measurements derived from observing the changes in O2 consumption with internal OPP have not been reported until now (see below).
COPR in pea and maize roots: O2 supplied only by gas-phase diffusion via the shoot.
Pea – indirect method
An example representative of the relationship found between ROL and shoot chamber OPPshoot is shown in Fig. 8A. At the lower OPPs a curvilinear relationship is evident and this was a consistent feature with all of the plants tested (19 in all). For most plants and as in the example shown, as shoot OPP increased further, a prominent inflexion of the ROL plot was usually identifiable and above this the plot of ROL vs. OPPshoot rose much more steeply. This is similar to the predictions made earlier and the inflexion is indicative of the cortical OPP in the root apex having reached a COPR. However, because of the limited number of gas mixtures used, the inflexion point could not always be pinpointed accurately and furthermore the ROL vs. OPPshoot relationship, although steeper at higher OPPs, was often still somewhat curvilinear (Fig. 8B), deviating from the ‘ideal’ model of Armstrong and Gaynard (1976) and suggesting perhaps an involvement of other oxidases. Consequently, if the COPR was to be determined from the ROL–OPPshoot relationship, the cause of these unexpected results had to be ascertained and, if possible, rectified. This was approached in various ways but the conclusions may be summarized as follows. The factors controlling ROL can be expressed in the following equation: ROL = ΔC/(RT + RS), where ΔC is the oxygen deficit between the source, Co, the moist air in the shoot chamber and Ce, the concentration at the electrode surface – at equilibrium this is effectively zero; RT is the apparent (or effective) resistance to O2 diffusion from the shoot (Armstrong, 1979); and RS is the diffusive resistance of the liquid shell between the root and electrode. At least three factors may contribute to the resistance RT: (1) the pore-space resistance, RP, (2) the respiratory consumption along the diffusion path and (3) radial O2 leakage from the root, other than that to the electrode. We were able to deduce that the pore space resistance of the root above the electrode would not change significantly as the root elongated and it is doubtful that changes in RP could account for any non-linearity in the ROL vs. OPPshoot relationship. Although the respiratory component of RT for a root of constant length should remain constant at OPPs above the COPR, any increase in root length during an experiment would cause a concomitant increase in total O2 uptake and increase the oxygen deficit between shoot and the root at the electrode position. As the experiments frequently lasted for 36 h, due to slow equilibration times, the primary pea root grew during this period and eventually protruded often by more than 1 cm below the electrode. Consequently, an increase in root oxygen demand associated with this root growth would effectively increase RT and might contribute to the non-linearity of the ROL plot. The third component of RT, that of O2 leakage (other than that to the electrode), may be categorized into (1) sub-apical leakage and (2) leakage from the root below the electrode. Although the sub-apical regions of the primary pea root are highly permeable to O2, a 1 % solid-agar jacket does restrict this leakage (Armstrong et al., 1983), and we initially assumed that leakage would be negligible. However, O2 leakage from the ‘unjacketed’ root below the electrode might be significant and change during the experiments. In conclusion, it seemed possible that root growth during the experiment might account for the non-linearity of the plots, a problem not encountered by Armstrong and Gaynard (1976) where highly aerenchymatous roots facilitated rapid equilibration after gas-mixture changes. By definition, a constant, maximum rate of respiration is attained at OPPs above the COPR and below the COPR respiration declines with decreasing OPP. If apparent respiratory rates could be calculated for the whole roots for each gas-mixture treatment, it might be anticipated that any marked change in respiratory activity and indicative of a COPR would be evident from the plot of respiratory rate vs. OPPshoot. The corresponding ROL could then be obtained from the experimental plot of ROL vs. OPPshoot, and subsequently the in vivo COPR could be calculated from the oxygen flux at that point. An equation was derived to determine the apparent respiratory demand for each OPPshoot (see Appendix) and this proved to be successful in establishing with certainty where the COPR inflexions point lay on the ROL vs. OPPshoot plots. For all plants the apparent respiration vs. OPPshoot curves took the bi-linear form shown in Fig. 9A and B, and each indicated an ROL value that identified the COPR. Based on these values the mean (± s.e.) root surface OPP for the 19 intact pea roots was 1·31 ± 0·13 kPa. Across the narrow non-porous epidermal/hypodermal root wall (approx. 30 µm) the estimated oxygen deficit due to wall respiration [taken as 120 ng cm−3 s−1 (Armstrong and Healy, 1984) and Dwall taken as 2·267 × 10−5 cm2 s−1] plus that due oxygen leakage through to the electrode amounted to only 0·23 kPa. The resulting cortical COPR, i.e. OPPwall plus the wall deficit, was 1·54 ± 0·12 kPa and the COPR range was 0·5–2·9 kPa. If a lower figure had been used for Dwall the estimated COPR would have been higher.
Fig. 8.
Examples of apical ROL versus OPPshoot and corresponding rates of apparent respiratory activity for roots of intact pea seedlings. In plots of type A the COPRs were calculated from the ROL values at the inflexion point; for those of type B extrapolations based on the inflexion point on the respiratory plot were used to estimate the ROL value at the inflexion point. Temperatures were approximately 23 °C.
Fig. 9.
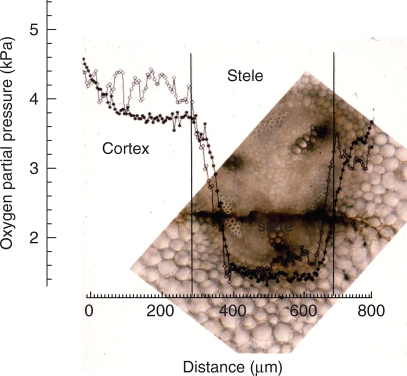
In-track (open circles) and out-track (closed circles) partial radial O2 profiles through the cortex and stele at 40 mm from the apex of a 90-mm-long pea root on a 5-d-old plant. The profiles overlay a photomicrograph of the root taken 24 h after penetration; the tracks taken by the O2 microelectrode are visible as a dark stain running from left to right through the pericycle phloem and one of the xylem arms of the triarch stele. Temperature was 21 °C.
It is very apparent, however, that the respiratory plots do not plateau above the COPR but continue to rise, albeit less steeply than below the COPR. We sought an explanation for this, and the likely effects of root growth on the ROL vs. OPPshoot plots, using an electrical analogue of the system (Armstrong and Wright, 1976). The results (Webb, 1982) are too voluminous to publish here but, in summary, they validated our treatment of the data (Appendix) and revealed that the slope in the respiratory curve above the inflexion point could be attributed to sub-apical O2 leakage into the agar cylinder rather than any real increase in root respiration. In the experiments on maize reported below, the roots were sleeved close-fitting glass tubes to minimize sub-apical leakage.
In view of the data of Berry and Norris (1949) and those from the mathematical modelling, the low COPR value derived from the ROL approach is indicative of respiratory decline at the centre of the stele/stelar meristem. Further support for this comes from an O2-microelectrode profile (Fig. 9) through the cortex and stele of a pea root obtained by Darwent (1997). As shown by the out-track, the OPP is relatively uniform across the cortex at approx. 3·8 kPa, but falls steeply within the stelar boundary as the electrode tracks across the pericycle and phloem, reaching a low of 1·7 kPa. The deficit, 2·1 kPa, is close to the range of COPR determined from the ROL data. Unfortunately, the track was tangential and did not cross the developing xylem. However, it did encompass the most active respiratory region and it is unlikely that the deficit would have been much greater had the track been strictly radial.
Maize – direct method
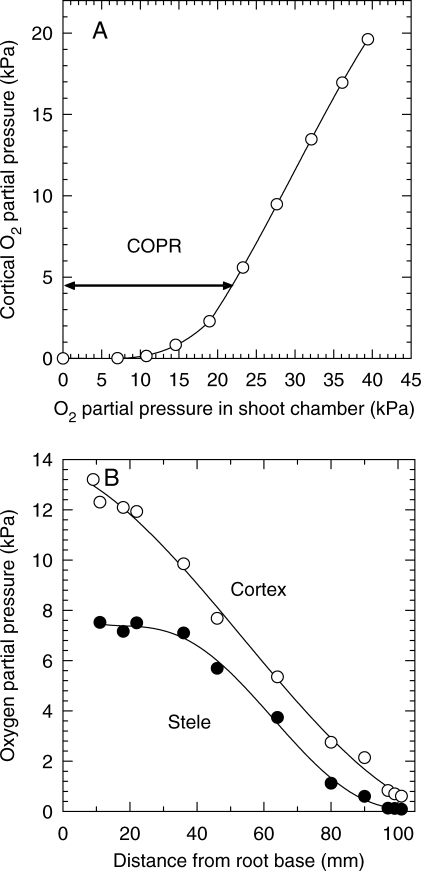
More gas-mixture changes than previously were made in these experiments, more certain methods were used to minimize sub-apical leakage and equilibration times were faster. Consequently, the difficulties experienced with pea were not as evident and results for the relationship between OPProot and OPPshoot (e.g. Fig. 10A) were as predicted (cf. Fig. 1, plot d). Nevertheless, it was difficult to establish precisely the inflexion point at which respiratory decline began. For the seven plants monitored by this direct method of measuring cortical OPPs, COPRs ranged from approx. 1·0 to 4·5 kPa (mean ± s.d., 2·3 ± 1·2 kPa). This is similar to the modelling prediction made earlier for the cortical OPP that corresponded with the onset of respiratory decline at the centre of the stele, although in that case the modelling was based on an O2 profile at 75 mm from the apex. However, the O2-microelectrode data for maize seedling roots (Darwent et al., 2003; Fig. 10B) showed that over the apical 60 mm of 100-mm roots, radial oxygen deficits across the stele might not significantly diminish until the OPP at the cortex–stelar boundary fell below 2 kPa. It should be emphasized that these data were for roots maintained at approx. 21 °C; at higher temperatures the COPRs would also be higher.
Fig. 10.
(A) Typical example of changes in equilibrium cortex OPPs versus OPPshoot in maize primary root. Root was 95 mm long and the O2-microelectrode was inserted at 5 mm from the tip. The COPR is indicated by the inflexion point (see arrow). Root had been grown in agar and probably had aerenchyma sub-apically. (B) Longitudinal OPP distribution in the cortex and stelar centre of a 6-d-old, 105-mm-long, non-aerenchymatous maize seedling root; shoot in air; temperature was 21 °C. After Darwent et al. (2003).
Of the two methods described here, the microelectrode (direct) has the obvious advantage in so far as no assumptions are necessary concerning the respiration and diffusivity in the epidermal/hypodermal cell layers. However, the use of microelectrodes as narrow as those required here to minimize damage to the root is not without problems. The electrodes are costly to make/buy, and are easily damaged: even root extension during the course of an experiment can be sufficient to cause breakages. Cylindrical electrodes, by contrast, are robust and do not damage the roots. For either method, however, precision in establishing the inflexion point of the ROL/cortical OPP versus shoot OPP will be depend upon the number of gas mixture changes made.
PERSPECTIVES AND CONCLUSIONS
In preparing this paper we had two aims: (1) to review and analyse the methodology-dependency of COPR, and (2) to make some judgement as to whether the roots of wetland species might be expected to have significantly different (and lower) COPRs than non-wetland ones. Regarding the latter, if one is concerned to learn whether COPR may be a determining factor in the tolerance of wetland conditions, then measurements must concentrate on sampling for COPR from cortical OPPs and preferably in the absence of an O2 source in the rooting medium. Previously, few measurements have been made in this way, but those that have, for example on rice and E. angustifolium (Armstrong and Gaynard, 1976), revealed values almost an order of magnitude lower than had at that time been found from most measurements relying on aeration from the bathing medium. The one prior exception was that of Greenwood and Goodman (1971) with excised mustard roots but in that case the low COPRs could be attributed to the narrowness of the roots and the minimized DBL thickness caused by high streaming rates in the bathing medium. However, even in the mustard roots, it is clear that, had the COPR been assessed from cortical OPPs, the values should have been even lower. Recent reports of very low root COPRs for a number of salt marsh species and cereals, including maize (Maricle and Lee, 2007), but again based on OPP detection in the bathing medium, and using excised and sealed whole root systems, are much less easy to explain. The results appear to run contrary to most previous reports (see earlier) and to the theoretical principles outlined in this paper and we believe that they should be treated with extreme caution; the highest COPR found, 1·6 kPa, was for the maize roots and yet the temperature was 28 °C. COPRs for Spartina anglica and S. alterniflora were <0·4 kPa despite the relatively wide, several layered non-porous outer cortex/hypodermis found in these species and which could be expected to result in particularly high COPRs when the OPP sampling is external to the root. These were not seedling roots and it may be that thin laterals dominated in the uptake of O2 and that barrier formation had minimized uptake by the parent adventitious roots. Nevertheless, for the reasons already given, meaningful COPR comparisons are not possible with data collected in this way.
From the modelling analyses we can conclude that the reasons why COPRs assessed from cortical OPPs can be so low are (1) the major terminal oxidase has the high O2-affinity characteristic of cytochrome oxidase, (2) it is only respiratory activity towards the centre of the stele/stelar meristem that is being limited at the COPR, (3) stelar diameters are small enough to ensure a relatively short radial diffusion path length between the sampling point and the point at which O2 limitation is first experienced, and (4) diffusive boundary layers and epidermal/hypodermal cell layers can exert no obfuscating influence on the measurements. All measurements here were made within the range 21–23 °C and higher values of COPR are to be expected at higher temperatures. The data that we have obtained for pea (COPR, 1·5 kPa) and maize (COPR, 1·9 kPa) are so similar to those of rice (COPR 2·4 kPa) and E. angustifolium (2 kPa) that we can see no reason at present for supposing that wetland and non-wetland plants might differ significantly in respiratory response to OPP in a way that could confer competitive advantage in wet, anaerobic, soils. Nevertheless, it is obvious that many more species need to be screened. Roots with large stelar diameters should tend to have higher COPRs and there is a tendency for wetland plants to have unusually small proportional and absolute stelar areas (McDonald et al., 2002; Colmer, 2003) that might be advantageous in wetland conditions and cause limitations for root function in non-waterlogged soil.
ACKNOWLEDGEMENTS
We thank Miss Sarah Lythe and Mr Vic Swetez for technical support and Dr Jean Armstrong for helpful comments on the manuscript.
APPENDIX
Pea roots and ROL
The equation from which apparent respiratory activities of the roots at different values of OPPshoot were derived was:
where M = the apparent respiratory activity of the root (g cm−3 s−1); Do = the diffusion coefficient of oxygen in air at the ambient temperature (cm2 s−1); ε = the fractional porosity of the root (0·038 for pea); Co = the oxygen concentration (g cm−3) at distance x = 0 (i.e. in the shoot chamber); Cx = the internal gas-phase O2 concentration (g cm−3) at the electrode position, x; Rp = the non-metabolic pore space resistance of the root between the shoot chamber and the electrode position (s cm−3) (obtainable from Armstrong et al., 1982); Ie = the O2 diffusion rate to the electrode (g s−1); l = the total length of root for each value of Co; and x = the root length from the base to the mid-point of the electrode.
LITERATURE CITED
- Armstrong W. Aeration in higher plants. In: Woolhouse HWW, editor. Advances in botanical research. Vol. 7. New York: Academic Press; 1979. pp. 225–332. [Google Scholar]
- Armstrong W, Beckett PM. Root aeration in unsaturated soil: a multi-shelled model of oxygen distribution and diffusion with and without sectoral blocking of the diffusion path. New Phytologist. 1985;100:293–311. [Google Scholar]
- Armstrong W, Beckett PM. Internal aeration and the development of stelar anoxia in submerged roots: a multishelled mathematical model combining axial diffusion of oxygen in the cortex with radial losses to the stele, the wall layers and the rhizosphere. New Phytologist. 1987;105:221–245. [Google Scholar]
- Armstrong W, Drew MC. Root growth and metabolism under oxygen deficiency. In: Waisel Y, Eshel A, Kafkafi U, editors. Roots the hidden half. 3rd edn. New York: Marcel Dekker Inc; 2002. pp. 729–761. [Google Scholar]
- Armstrong W, Gaynard TJ. The critical oxygen pressure for root respiration in intact plants. Physiologia Plantarum. 1976;37:200–206. doi: 10.1111/j.1399-3054.1976.tb03958.x. [DOI] [PubMed] [Google Scholar]
- Armstrong W, Healy MT. Oxygen diffusion in pea. III. Changes in the oxygen status of the primary pea root attributable to an ageing of the tissues. New Phytologist. 1984;96:179–185. [Google Scholar]
- Armstrong W, Webb T. A critical oxygen pressure for root extension in rice. Journal of Experimental Botany. 1985;36:1573–1582. [Google Scholar]
- Armstrong W, Wright EJ. Radial oxygen loss from roots: the theoretical basis for the manipulation of flux data obtained by the cylindrical platinum electrode technique. Physiologia Plantarum. 1975;35:21–26. [Google Scholar]
- Armstrong W, Wright EJ. An electrical analogue to simulate the oxygen relations of roots in anaerobic media. Physiologia Plantarum. 1976;36:383–387. [Google Scholar]
- Armstrong W, Cousins D, Armstrong J, Turner DW, Beckett PM. Oxygen distribution in wetland plant roots and permeability barriers to gas-exchange with the rhizosphere: a microelectrode and modelling study with Phragmites australis. Annals of Botany. 2000;86:687–703. [Google Scholar]
- Armstrong W, Healy MT, Webb T. Oxygen diffusion in pea. I. Pore-space resistance in the primary root. New Phytologist. 1982;91:647–659. [Google Scholar]
- Armstrong W, Healy MT, Lythe S. Oxygen diffusion in pea. II. The oxygen status of the primary root as affected by growth, the production of laterals and radial oxygen loss. New Phytologist. 1983;94:549–559. [Google Scholar]
- Armstrong W, Beckett PM, Justin SHFW, Lythe S. Modelling and other aspects of root aeration. In: Jackson MB, Davies DD, Lambers H, editors. Plant life under oxygen stress. The Hague: SPB Academic Publishing; 1991. pp. 267–282. [Google Scholar]
- Begg CBM, Kirk GJD, MacKenzie AF, Neue HU. Root-induced iron oxidation and pH changes in the lowland rice rhizosphere. New Phytologist. 1994;128:469–477. doi: 10.1111/j.1469-8137.1994.tb02993.x. [DOI] [PubMed] [Google Scholar]
- Berry LJ. The influence of oxygen tension on the respiratory rate in different segments of onion roots. Journal of Cell & Comparative Physiology. 1949;33:41–66. doi: 10.1002/jcp.1030330105. [DOI] [PubMed] [Google Scholar]
- Berry LJ, Brock MJ. Polar distribution of respiratory rate in onion root tip. Plant Physiology. 1946;21:542–549. doi: 10.1104/pp.21.4.542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry LJ, Norris WE. Studies of onion root respiration. l. Velocity of oxygen consumption in different segments of root at different temperatures as a function of partial pressure of oxygen. Biochimica et Biophysica Acta. 1949;3:593–606. [Google Scholar]
- van Bodegom P, Goudriaan J, Leffelaar P. A mechanistic model on methane oxidation in a rice rhizosphere. Biogeochemistry. 2001;55:145–177. [Google Scholar]
- Darwent MJ. University of Hull; 1997. The development and use of microelectrodes for the study of oxygen transport and distribution in roots. PhD thesis. [Google Scholar]
- Darwent MJ, Armstrong W, Armstrong J, Beckett PM. Exploring the radial and longitudinal aeration of primary maize roots by means of Clark-type oxygen microelectrodes. Russian Journal of Plant Physiology. 2003;50:722–732. [Google Scholar]
- Colmer TD. Long-distance transport of gases in plants: a perspective on internal aeration and radial oxygen loss from roots. Plant, Cell and Environment. 2003;26:17–36. [Google Scholar]
- Garthwaite AJ, Armstrong W, Colmer TD. Assessment of the O2 diffusivity across the barrier to radial O2 loss in adventitious roots of Hordeum marinum. New Phytologist. 2008;179:405–416. doi: 10.1111/j.1469-8137.2008.02467.x. [DOI] [PubMed] [Google Scholar]
- Geigenberger P, Fernie AR, Gibon Y, Christ M, Stitt M. Metabolic activity decreases as an adaptive response to low internal oxygen in growing potato tubers. Biological Chemistry. 2000;381:723–740. doi: 10.1515/BC.2000.093. [DOI] [PubMed] [Google Scholar]
- Gibbs J, Turner DW, Armstrong W, Darwent MJ, Greenway H. Solute transport and water relations of oxygen deficient maize roots. I. Development of O2-deficiency in the stele reduces radial solute transport to the xylem. Australian Journal of Plant Physiology. 1998;25:745–758. [Google Scholar]
- Gilbert B, Frenzel P. Rice roots and methane oxidation: the activity of bacteria, their distribution and the microenvironment. Soil Biology and Biochemistry. 1998;30:1903–1916. [Google Scholar]
- Gonzhlez Siso MI, Ramil E, Cerdhn ME, Freire-Picas MA. Respirofermentative metabolism in Kluyveromyces lactis: ethanol production and the Crabtree effect. Enzyme and Microbial Technology. 1996;18:585–591. doi: 10.1016/s0141-0229(00)00161-7. [DOI] [PubMed] [Google Scholar]
- Greenwood DJ. Effect of oxygen distribution in the soil on plant growth. In: Whittington WJ, editor. Root growth. London: Butterworths; 1968. pp. 202–223. [Google Scholar]
- Greenwood DJ, Goodman D. Studies on the oxygen supply to the roots of mustard seedlings (Sinapsis alba L.) New Phytologist. 1971;70:85–96. [Google Scholar]
- Jensen SI, Kühl M, Glud RN, Jørgensen LB, Priemé A. Oxic microzones and radial oxygen loss from roots of Zostera marina. Marine Ecology Progress Series. 2005;293:49–58. [Google Scholar]
- Justin SHFW, Armstrong W. The anatomical characteristics of roots and plant response to soil flooding. New Phytologist. 1987;106:465–495. [Google Scholar]
- Kirk GJD, Kronzucker HJ. The potential for nitrification and nitrate uptake in the rhizosphere of wetland plants: a modelling study. Annals of Botany. 2005;96:639–646. doi: 10.1093/aob/mci216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy H, Schade AL, Bergmann L, Harris S. Studies in the respiration of the white potato. II Terminal oxidase system for potato tuber respiration. Archives of Biochemistry. 1948;19:273–286. [PubMed] [Google Scholar]
- Longmuir IS. Respiration rate of bacteria as a function of O2 concentration. Biochemical Journal. 1954;57:81–87. doi: 10.1042/bj0570081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luxmoore RJ, Stolzy LH, Letey J. Oxygen diffusion in the soil–plant system. II. Respiration rate, permeability and porosity of consecutive excised segments of maize and rice roots. Agronomy Journal. 1970;62:322–324. [Google Scholar]
- McDonald MP, Galwey NW, Colmer TD. Similarity and diversity in adventitious root anatomy as related to root aeration among a range of wetland and dryland grass species. Plant, Cell and Environment. 2002;25:441–451. [Google Scholar]
- Maricle BR, Lee RW. Root respiration and oxygen flux in salt marsh grasses from different elevational zones. Marine Biology. 2007;151:413–423. [Google Scholar]
- Otterstedt K, Larsson C, Bill RM, Stǻhlberg A, Boles E, Hohmann S, Gustafsson L. Switching the mode of metabolism in the yeast Saccharomyces cerevisiae. European Molecular Biology Organization Reports. 2004;5:532–537. doi: 10.1038/sj.embor.7400132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saglio PH, Rancillac F, Bruzan F, Pradet A. Critical oxygen for growth and respiration of excised and intact roots. Plant Physiology. 1984;76:15l–154. doi: 10.1104/pp.76.1.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scotter DR, Thurtell GW, Tanner CB. Measuring oxygen uptake by the roots of intact plants under controlled conditions. Soil Science. 1967;104:374–378. [Google Scholar]
- Sorrell BK, Mendelssohn IA, McKee KL, Woods RA. Ecophysiology of wetland plant roots: a modelling comparison of aeration in relation to species distribution. Annals of Botany. 2000;86:675–686. [Google Scholar]
- Thiman KV, Yocum CS, Hackett DP. Terminal oxidases and growth in plant tissue. III. Terminal oxidation in potato tuber tissue. Archives of Biochemistry and Biophysics. 1954;53:239–257. doi: 10.1016/0003-9861(54)90249-0. [DOI] [PubMed] [Google Scholar]
- Thomson CJ, Greenway H. Metabolic evidence for stelar anoxia in maize roots exposed to low O2 concentrations. Plant Physiology. 1991;96:1294–1301. doi: 10.1104/pp.96.4.1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb T. University of Hull; 1982. Some aspects of root growth, respiration and internal aeration in wetland and non-wetland species. PhD thesis. [Google Scholar]