Abstract
Background and Aims
An investigation was carried out to determine whether stomatal closure in flooded tomato plants (Solanum lycopersicum) results from decreased leaf water potentials (ψL), decreased photosynthetic capacity and attendant increases in internal CO2 (Ci) or from losses of root function such as cytokinin and gibberellin export.
Methods
Pot-grown plants were flooded when 1 month old. Leaf conductance was measured by diffusion porometry, the efficiency of photosystem II (PSII) was estimated by fluorimetry, and infrared gas analysis was used to determine Ci and related parameters.
Key Results
Flooding starting in the morning closed the stomata and increased ψL after a short-lived depression of ψL. The pattern of closure remained unchanged when ψ`L depression was avoided by starting flooding at the end rather than at the start of the photoperiod. Raising external CO2 concentrations by 100 µmol mol−1 also closed stomata rapidly. Five chlorophyll fluorescence parameters [Fq′/Fm′, Fq′/Fv′, Fv′/Fm′, non-photochemical quenching (NPQ) and Fv/Fm] were affected by flooding within 12–36 h and changes were linked to decreased Ci. Closing stomata by applying abscisic acid or increasing external CO2 substantially reproduced the effects of flooding on chlorophyll fluorescence. The presence of well-aerated adventitious roots partially inhibited stomatal closure of flooded plants. Allowing adventitious roots to form on plants flooded for >3 d promoted some stomatal re-opening. This effect of adventitious roots was not reproduced by foliar applications of benzyl adenine and gibberellic acid.
Conclusions
Stomata of flooded plants did not close in response to short-lived decreases in ψL or to increased Ci resulting from impaired PSII photochemistry. Instead, stomatal closure depressed Ci and this in turn largely explained subsequent changes in chlorophyll fluorescence parameters. Stomatal opening was promoted by the presence of well-aerated adventitious roots, implying that loss of function of root signalling contributes to closing of stomata during flooding. The possibility that this involves inhibition of cytokinin or gibberellin export was not well supported.
Key words: Root to shoot communication, flooding stress, stomatal closure, photosynthesis, chlorophyll fluorescence, gas exchange, adventitious roots, plant hormones, abscisic acid, cytokinins, gibberellic acid
INTRODUCTION
Soil flooding is a major abiotic stress that damages many agricultural crops and also poorly adapted plants in more natural environments (Jackson, 2004, 2006). One of the earliest responses is a reduced ability of roots of sensitive species such as tomato to take up water compared with well-drained plants (Kramer, 1969; Schildwacht, 1989; Else et al., 1995, 2001). This response can start within 2–6 h and is the outcome of decreasing root hydraulic conductance (Lp) thought to result from a disruption of aquaporin functioning by the cytosolic acidosis that anoxia brings about (Tournaire-Roux et al., 2003). A lowered Lp could trigger subsequent daytime shoot water deficits but, in flooded tomato, protection is afforded by partial stomatal closure that commences after a short delay.
The causes of stomatal closure in flooded tomato plants remain obscure. The response is presumed to be the outcome of root to shoot signalling (reviewed in Jackson, 2002). Signals may be positive, negative or accumulative in character depending on whether the message comprises (a) increased export of a promoter from roots to shoots; (b) decreased export of an inhibitor of opening; or (c) an accumulation of promoter in leaves resulting from decreased demand by roots. Previous work using standard infrared gas analyser (IRGA)-based gas exchange analysis indicated that, in addition to closing stomata, flooding may damage the photosynthetic apparatus (Moldau, 1973; Bradford, 1983a, b). Both effects will slow photosynthesis (e.g. Guidi and Soldatini, 1997), with implications for concentrations of intercellular CO2 (Ci), a potent promoter of stomatal closure (Mott 1988). A decsion was therefore made to re-examine the relationship between flooding, stomatal closure and photosynthesis using conventional gas exchange, diffusion porometry and chlorophyll a fluorescence to analyse the operational status of photosystem II (PSII). It was known from earlier work (Janowiak et al., 2002) that flooding can limit the quantum efficiency of PSII in tomato, and Ahmed et al. (2006) reported a similar response in mung bean. To test the involvement of negative root to shoot signalling in these responses, an investigation was also conducted to determine if a well-aerated adventitious root system or applications of cytokinin and gibberellin could offset the effects of flooding on stomatal closure. This latter work extends several previous studies (Jackson and Campbell, 1979; Bradford, 1983a; Wadman-van-Schravendijk and van Andel, 1985).
MATERIALS AND METHODS
Plant material and growth conditions
Seeds of tomato (Solanum lycopersicum ‘Ailsa Craig’) were sown into a mixture of peat, sand and clay (2 : 1 : 1, by volume). After 2 weeks, seedlings were replanted into pots (130 × 130 × 110 mm) filled with similar compost. For most tests, plants were maintained in growth rooms with a light/dark temperature of 25/20 °C and a 16 h photoperiod (0700 h–2300 h) provided by Osram Vialox lamps, the light intensity at plant height being 200 µmol m−2 s−1. In Fig. 1, a 12 h photoperiod was used. Relative humidity (RH) was not controlled but averaged between 40 and 50 %. Plants were watered regularly throughout each day and with Hoagland's nutrient solution on alternate days. Side shoots were removed regularly. The position of the plants in the growth room was changed weekly to reduce any effects of localized variation in light intensity and air temperature. Plants were used at the 7- to 8-leaf stage and were divided into well-drained and flooded treatments at random. Unless otherwise indicated, plant root systems were flooded at approx. 0900 h by placing the pots of soil into larger pots filled with tap water, warmed to 25 °C and maintained 10 mm above compost level. Well-drained plants were watered regularly throughout each photoperiod to a pre-determined weight representing pot capacity. There were eight replicate plants per treatment.
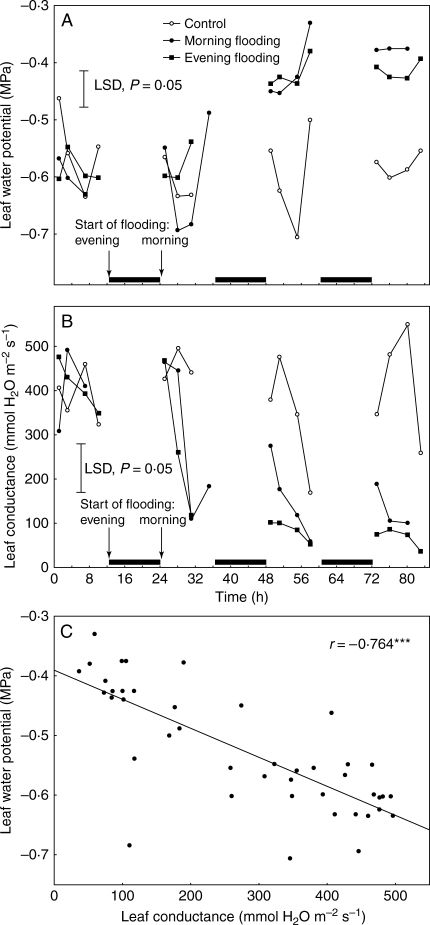
Fig. 1.
Effects of flooding the soil for up to 83 h on (A) leaf water potential and (B) leaf conductance of the third and fourth oldest leaves of 1-month-old tomato plants. Flooding was started either at the start or at the end of a photoperiod. Linear correlation between leaf conductance and leaf water potential is given in (C). Each point in (A) and (B) represents the mean of seven replicates. Vertical lines are LSDs at P = 0·05. Black boxes on the x-axis indicate dark periods.
To test the effect of well-aerated roots on responses to flooding, an extensive adventitious root system was formed approx. 10 cm above the original root system during the 2 weeks before flooding. The roots were induced by surrounding the stem with regularly moistened peat retained in a 50 mm deep circular dish supported by plastic pillars much as described by Jackson and Campbell (1979). Plants with adventitious roots were compared with those where adventitious roots were removed just before flooding. In some experiments, plants were given a foliar spray of deionized water containing 0·05 % (v/v) Tween-20 surfactant, and gibberellic acid (GA) and benzyladenine (BA) at 10 mg L−1 previously dissolved in a minimum of ethanol as co-solvent. Controls were spayed with deionized water containing 1 % (v/v) ethanol and Tween-20 alone. Plants were sprayed to run-off 24 h before the start of flooding and again each day for a further 2 d.
Adjusting external carbon dioxide concentrations
In experiments where the external CO2 concentration (Ca) was adjusted, plants were transferred the previous day to a 1·2 × 0·7 × 1 m chamber constructed from clear acrylic (‘Perspex’) sheeting. Preliminary experiments determined the day and night temperature set points in the growth room necessary to maintain the air temperature within the closed Perspex chamber at 25/20 °C. The humidity within the sealed chamber containing the plants was kept between 60 and 75 % by circulating the air through a cold trap and a container of silica gel. Air was circulated within the chamber using an electric fan, and air temperature and RH were monitored every 5 s using temperature and RH sensors (KWAP SA, Krakow, Poland). Data were averaged over 5 min intervals and logged using a MPI-LAB data scanner and Rejestrator monitoring software (Metronic Instruments, Krakow, Poland).
Preliminary experiments showed that approx. 800–1000 ppm CO2 could be retained within the Perspex chamber for many hours, confirming that the sealed chamber was gas tight. In the light, Ca was maintained inside the chamber by allowing the plants to deplete ambient CO2 to approx. 260 µmol mol−1 then raising the concentration by approx. 100 µmol mol−1 using pure CO2 from a gas cylinder (OZON, Krakow, Poland). Such additions were necessary at 50 min intervals throughout each light period to bring concentrations back to approx. 360 µmol mol−1. A CO2 sensor (GMT220, Vaisala, Helsinki, Finland) placed inside the Perspex chamber recorded changes in CO2 every 5 s to generate data that were averaged over 5 min intervals and logged using a MPI-LAB data scanner and ‘Rejestrator’ monitoring software. During each dark period, Carbosorb (20 g) was placed inside the sealed chamber to absorb the CO2 released by respiration and to maintain Ca close to 360 µmol mol−1. Each morning, the chamber was opened briefly at 0800 h and the Carbosorb removed.
Reduced Ca in the Perspex chamber was achieved by allowing the plants to deplete the [CO2] to 160 µmol mol−1 before raising levels by approx. 200 µmol mol−1 using injections of pure CO2. Frequent additions of CO2 maintained the Ca between 160 and 360 µmol mol−1 throughout two photoperiods. In a separate experiment, Ca was increased gradually over 3 h from 260–460 µmol mol−1 (on average 360 µmol mol−1) to 360–560 µmol mol−1 (on average 460 µmol mol−1) and maintained within this higher range for the rest of the photoperiod.
In experiments where Ca was modified, the fluorimeter and electronic balance used for transpiration measurements were sealed inside the Perspex chamber with the plants. Measurements of chlorophyll a fluorescence and whole-plant water loss were made at intervals throughout each photoperiod.
Leaf conductance, leaf water potentials, gas exchange and transpiration rates
Leaf conductances (gs) of the youngest fully expanded leaf (usually the fifth leaf, counting from the base) of flooded and well-drained plants were determined approximately every 2 h during the photoperiod using a hand-held, AP4 porometer (Delta-T Devices, Cambridge, UK). Leaf water potentials (ψL) were measured with a Scholander-type pressure chamber using freshly excised leaves previously used for estimating leaf conductances.
CO2 assimilation was measured with a Ciras-1 portable IRGA photosynthesis system (PP Systems, Hitchen, Herts, UK) in conjunction with a Ciras PLC cuvette (broad window 2·5 cm2) warmed to 25 °C. Incident PAR was supplied by a halogen light cuvette unit set at 700 µmol m−2 s−1 which preliminary measurements had shown to saturate photosynthesis. These parameters were used for all subsequent measurements. Ciras remote control software was used to determine the relationship between assimilation rate (A) and Ci over a range of CO2 concentrations, varying Ca using the Ciras modified CO2 regulator system. CO2 concentration was controlled automatically to pre-set levels. Measurements started with ambient, and then the CO2 concentration was reduced below ambient, returning to ambient prior to elevating to saturation levels at 1500 µmol mol−1. A/Ci curves (not shown) generated from well-drained and flooded plants were used to calculate Ci, A, Amax, carboxylation efficiencies (dA/dCi) and the CO2 compensation point. Gravimetric measurements of whole-plant transpiration to within 0·1 g, corrected for evaporation from the compost surface, were made at 2 h intervals during each photoperiod using an electronic balance (RADWAG, Radom, Poland).
Chlorophyll a fluorescence
Chlorophyll fluorescence was measured with a PAM 2000 fluorimeter (Heinz Walz, Effeltrich, Germany) using standard instrument settings (saturating pulse of 12 000 µmol m−2 s−1 for 0·8 s) with additional far red light (735 nm) to enable estimation of ground state fluorescence (F0′) (Walz 1993). The estimates of F0′ were identical to those calculated using formulae suggested by Oxborough and Baker (1997). The fluorescence terminology used by Baker et al. (2001) and Lawson et al. (2002) has been adopted here. In light-adapted plants, the maximum fluorescence of dark-adapted leaves after a flash of saturating light (Fm′), and levels of fluorescence during a short light-saturating pulse at a point between F0′ and Fm′(F′) were measured and used to estimate the effective quantum efficiency of PSII photochemistry (Fq′/Fm′) [Fq′/Fm′ = (Fm′ – F′)/Fm′)], photochemical fluorescence quenching (Fq′/Fv′) [Fq′/Fv′ = (Fm′ – F′)/(Fm′ – F0′)] and the operating efficiency of PSII photochemistry (Fv′/Fm′) [Fv′/Fm′ = (Fm′ – F0′)/Fm′)]. In dark-adapted plants, F0 and also levels of fluorescence measured at a very low photosynthetic photon flux density (PPFD) of <1 µmol m−2 s−1 and during a short light-saturating pulse (Fm) were measured and used to estimate the maximum quantum yield of PSII when fully oxidized (Fv/Fm) [Fv/Fm = Fm – F0/Fm]. Non-photochemical fluorescence quenching (NPQ) [(Fm – Fm′)/(Fm – F0′)] was calculated utilizing measurements made on both dark-adapted and light-adapted leaves.
Detached leaflet experiments
Solutions of abscisic acid (ABA) prepared using ethanol as co-solvent were applied to detached leaves to close their stomata as follows. Ten leaflets from the fifth and sixth oldest leaves of well-drained plants were excised under a stream of deionized water, the petioles re-cut under water and inserted through holes in the lids of Eppendorf vials into a solution of 1 % ethanol (v/v). Leaflets in their vials were left to transpire, and weight loss, recorded after 1 h, was used as an estimate of relative stomatal apertures. F′, Fm′ and F0′ were measured in each leaflet at the beginning and end of the hour. Five of the leaves were then transferred quickly to similar vials containing a solution of (+)-ABA (50 µmol m−3 in 1 % ethanol); the other five were transferred quickly to vials containing a solution of 1 % ethanol. Weight loss and fluorescence parameters were then recorded hourly for a further 4 h. Growth room lights were then turned off, and weight loss, F0 and Fm were recorded 1 h later. Leaf areas were determined with image analysis software (Delta-T Devices, Cambridge, UK).
Statistical analyses
Fully randomized experimental designs were used. Data are mean values of seven or eight plants (stomatal conductance and leaf gas exchange) or 24 fluorescence measurements from eight plants. Detached leaflet data are mean values of five replicate leaflets. In experiments where Ca was altered, fluorescence values are means of eight replicate leaflets. Least significant differences (LSDs) were calculated (P = 0·05) and are presented in the figures. Standard errors were calculated and Student′s t-tests applied where appropriate.
RESULTS
Effects on leaf water potentials, stomatal closure and transpiration
When flooding began within the first hour of a 12 h photoperiod, leaf water potentials (ψL) decreased from –0·55 MPa to approx. –0·80 MPa within 4–7 h but then recovered to exceed those of well-drained plants towards the end of the photoperiod. This indicates a mild and temporary leaf dehydration followed by rehydration to levels that exceed control values. In the second and third photoperiods, the ψL of flooded plants remained substantially above that of well-drained plants at all times (Fig. 1A). This rapid and sustained rehydration of the leaves of flooded plants was associated with an equally prompt and sustained partial closing of the stomata, as indicated by substantial decreases in leaf conductance to water vapour loss (gs; Fig. 1B). These responses were also examined in plants where flooding began at the end of the preceding photoperiod rather than at its start. A previous study (Bradford and Hsaio, 1982) indicated that evening flooding changes the relationship between gs and ψL. Although no substantial difference was seen in the patterns of stomatal response, the temporary decline of ψL early in the first photoperiod after flooding was eliminated if flooding was started the previous evening. A statistically significant linear correlation between ψL and gs over the entire 81 h long experiment (Fig. 1C) illustrates the close association between stomatal closure (gs) and increase in ψL.
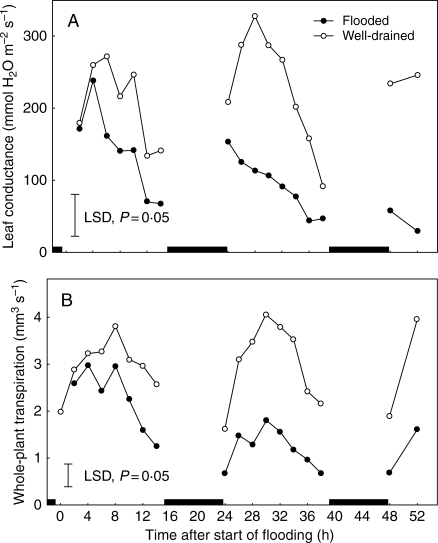
In all further work, flooding was started in the morning. A detailed time course of the stomatal response of plants to morning flooding is given in Fig. 2A. This reveals more clearly the pattern of flooding-induced closure of stomata during the first and subsequent photoperiods. The closely associated slowing of transpiration (Fig. 2B) reinforces the water-conserving impact of closure that results in the raising of ψL shown in Fig. 1. It also demonstrates that transpiration rates can act as a proxy for stomatal closure under the conditions used here. This was used to demonstrate the ability of increasing the CO2 concentrations to close stomata (Table 1), CO2 being a possible signal for closure of stomata of flooded plants. Earlier determinations of Ci over a range of Ca using IRGA analysis showed that daytime Ci would be increased from 195 to approx. 244 µmol mol−1 when Ca is increased to exceed the approx. 360 µmol mol−1 normal atmospheric concentration by 100 µmol mol−1. A rise in Ca of this magnitude slowed transpiration by up to 30 %, an effect similar to that induced by flooding. In plants with stomata already closing in response to flooding, raising the Ca had no additional effect (Table 1).
Fig. 2.
The effect of soil flooding for up to 52 h on (A) leaf conductance of the fifth oldest leaf and (B) whole-plant transpiration rates in 1-month-old tomato plants. Each point represents the mean of eight replicates. Vertical lines are LSDs at P = 0·05. Black boxes on the x-axis indicate dark periods.
Table 1.
The effect of increasing Ca from 360 to 460 µmol mol−1 on the transpiration rate (mm3 s−1) of well-drained and flooded tomato plants
| Well-drained |
Flooded |
|||
|---|---|---|---|---|
| Time (h) | 360 µmol mol−1 | 460 µmol mol−1 | 360 µmol mol−1 | 460 µmol mol−1 |
| 0 | 1·11 ± 0·19 | – | – | – |
| 2 | 1·50 ± 0·14 | 1·67 ± 0·19 | 1·53 ± 0·14 | 1·25 ± 0·32 |
| 4 | 1·67 ± 0·36 | 1·53 ± 0·14 | 1·53 ± 0·14 | 1·39 ± 0·28 |
| 6 | 2·08 ± 0·14 | 1·67 ± 0·19* | 1·39 ± 0·27 | 1·53 ± 0·14 |
| 8 | 2·01 ± 0·21 | 1·67 ± 0·19* | 1·67 ± 0·19 | 1·39 ± 0·28 |
| 10 | 1·94 ± 0·28 | 1·39 ± 0·19* | 1·53 ± 0·14 | 1·25 ± 0·14 |
Plants were flooded at 0900 h; Ca was increased gradually over 3 h from 360 to 460 µmol mol−1 and maintained within this higher range for the rest of the first photoperiod. Whole-plant transpiration rates were measured gravimetrically. Values are means of three replicate plants ± s.e.; asterisks indicate significant differences between CO2 treatments (P = 0·05).
Effects of soil flooding and ABA-induced stomatal closure on chlorophyll fluorescence
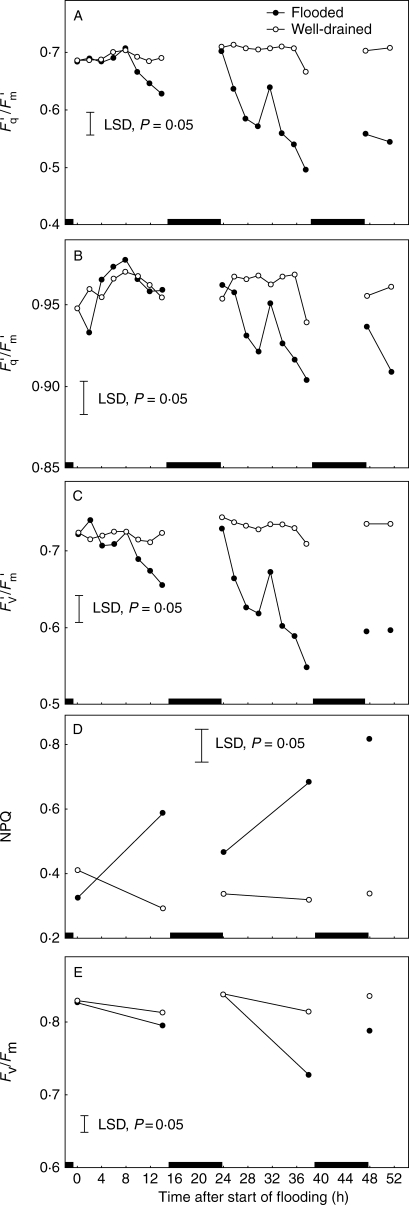
Five different but inter-related parameters of chlorophyll fluorescence were quantified (Fq′/Fm′, Fq′/Fv′, Fv′/Fm′, NPQ and Fv/Fm) in attempts to identify the earliest and the most persistent indicators of any effects of flooding on PSII functionality. In well-drained plants, Fq′/Fm′ remained reasonably constant throughout each photoperiod. However, it was reduced significantly by flooding, the effect starting about 6 h after stomata began to close and growing stronger during the 52 h long experiment (Fig. 3A) as stomata closed more completely (compare Fig. 2A with Fig. 3A). The early decreases in Fq′/Fm′ were closely associated with decreases in Fv′/Fm′. Flooding also reduced Fq′/Fv′ but not until the second photoperiod in the present experiment (Fig. 3B). In other tests (results not shown), timing of decreases in Fq′/Fv′ tracked those of Fq′/Fm′ more closely. The amount of NPQ (Fig 3D) was increased by flooding. The effect was clear by the end of the first photoperiod and increased during the two subsequent photoperiods, but with a notable degree of recovery over each dark period. Fv/Fm was smaller at the beginning, compared with the end, of each dark period in both flooded and well-drained plants (Fig. 3E). Within this diurnal range, Fv/Fm was slightly depressed in flooded plants when measured after 14 h of flooding but recovered during the night (Fig. 3E). However, by the end of the second photoperiod, Fv/Fm was strongly depressed in flooded plants (Fig. 3E). In other experiments (data not shown), Fv/Fm values for flooded plants remained significantly lower than in their well-drained counterparts throughout the third and fourth photoperiods.
Fig. 3.
The effect of soil flooding for up to 52 h on (A) quantum efficiency of PSII photochemistry (Fq′/Fm′), (B) photochemical fluorescence quenching (Fq′/Fv′), (C) the operating efficiency of PSII photochemistry (Fv′/Fm′), (D) non-photochemical fluorescence quenching (NPQ) and (E) maximum quantum efficiency of PSII photochemistry (Fv/Fm). Plants were dark-adapted for approx. 20 min prior to measurement of F0 and Fm to estimate NPQ and Fv/Fm at the end of each photoperiod. Each point represents a mean of 24 replicate measurements made on the fifth oldest leaves of eight 1-month-old plants per treatment. Vertical lines are LSDs at P = 0·05. Black boxes on the x-axis indicate dark periods.
ABA treatment was used to close stomata quickly and check the extent to which the effects of flooding on chlorophyll fluorescence were seemingly the outcome of partially closed stomata. The rate of water loss (marker for stomatal conductance) was reduced after 2 h of transferring excised leaflets to solutions containing 50 µmol m−3 (+)-ABA [from 1·71 ± 0·04 (s.e) mmol H2O m−2 s−1 to 0·56 ± 0·06 mmol H2O m−2 s−1, n = 5]. Fq′/Fm′, the most flooding-responsive fluorescence parameter, was reduced by a statistically significant 20 % from 0·69 ± 0·04 to 0·52 ± 0·02 (n = 5). This effect was retained when measurements were made again 2 h later.
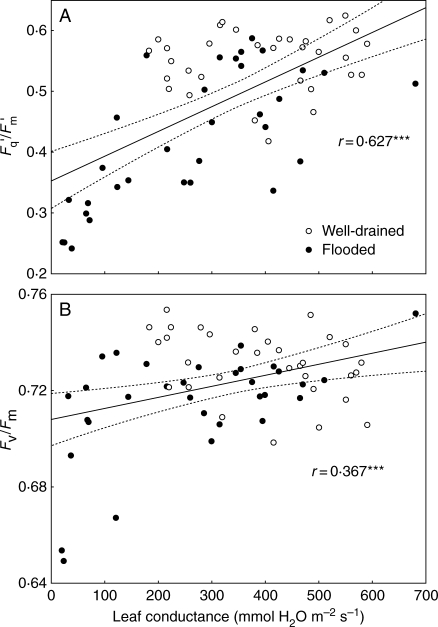
In a separate flooding experiment, many coincidental measurements of Fq′/Fm′ and leaf conductances were made. Correlation analysis (Fig. 4A) shows a close association between leaf conductance and Fq′/Fm′ over 3 d. A much weaker relationship between leaf conductance and Fv/Fm (Fig. 4B) reflects a longer delay between the first closing of stomata and Fv/Fm depression. This resulted in some plants with small gs but high values of Fv/Fm during the first half of the flooding treatment.
Fig. 4.
Linear correlations between leaf conductances and (A) quantum efficiency of PSII photochemistry (Fq′/Fm′) or (B) maximum quantum efficiency of PSII photochemistry (Fv/Fm) during 3 d flooding of 1-month-old tomato plants. Points are from paired individual readings taken over 3 d of flooding. R is the correlation coefficient, and dashed lines show the confidence range at P = 0·05.
IRGA analysis of leaf gas exchange and CO2 effects on fluorescence parameters
The consequences of decreased leaf conductances and of other possible effects of flooding on photosynthesis and Ci were assessed over 28 h of flooding using an IRGA. Flooding for 5–28 h was found to reduce Ci in association with stomatal closure (smaller gs) (Table 2). Assimilation rates of CO2 (A and Amax) in leaves of flooded plants also decreased during the first few hours of flooding but did not drop below those of well-drained plants to a statistically significant extent (P = 0·05) until after 24–28 h of flooding (Table 2), by which time the effects were substantial. Rates of carboxylation efficiency (dA/dCi) were also reduced but not until the second day of soil flooding. This was accompanied by a rise in the CO2 compensation point (Γ) of almost 40 % (Table 2).
Table 2.
Effect of up to 28 h flooding of the soil on leaf gas exchange of 1-month-old tomato plants
| Treatment | A (μmol m−2 s−1) | Amax (μmol m−2 s−1) | Ci (μmol mol−1) | gs (mmol m−2 s−1) | dA/dCi (μmol m−2 s−1) | Γ (μmol mol−1) |
|---|---|---|---|---|---|---|
| WD 0–1 h | 11·18 ± 1·22 | 20·74 ± 1·28 | 181·75 ± 7·59 | 196·50 ± 26·53 | 0·073 ± 0·005 | 63·63 ± 2·40 |
| FL 1–2 h | 11·63 ± 0·72 | 22·30 ± 1·76 | 190·00 ± 2·66 | 222·50 ± 30·95 | 0·088 ± 0·008 | 62·73 ± 3·60 |
| WD 2–3 h | 10·11 ± 0·59 | 19·87 ± 1·67 | 195·57 ± 3·74 | 196·00 ± 7·07 | 0·075 ± 0·007 | 64·96 ± 2·14 |
| FL 3–4 h | 10·16 ± 1·12 | 18·31 ± 2·50 | 188·43 ± 4·90 | 194·86 ± 33·31 | 0·076 ± 0·007 | 61·37 ± 4·68 |
| WD 4–5 h | 10·84 ± 0·69 | 19·81 ± 1·92 | 197·86 ± 4·17 | 221·43 ± 18·97 | 0·077 ± 0·007 | 62·19 ± 2·94 |
| FL 5–6 h | 10·01 ± 0·65 | 16·77 ± 1·69 | 178·43* ± 4·15 | 165·00* ± 23·1 | 0·087 ± 0·005 | 66·12 ± 3·25 |
| WD 24–28 h | 11·27 ± 0·82 | 19·37 ± 1·39 | 198·27 ± 5·42 | 232·55 ± 22·35 | 0·097 ± 0·005 | 93·49 ± 9·21 |
| FL 24–28 h | 4·95* ± 0·58 | 13·88* ± 2·03 | 175·36* ± 6·38 | 69·00* ± 6·51 | 0·058* ± 0·009 | 129·85* ± 21·21 |
Measurements were made on the fifth oldest leaf using a Ciras-1 portable IRGA photosynthesis system. A/Ci curves were generated for well-drained and flooded plants and were used to calculate internal Ci, A, Amax, carboxylation efficiencies (dA/dCi) and the CO2 compensation point (Γ). Estimates of stomatal conductance (gs) were also made with the Ciras system. Results are means of 7–8 replicates with associated s.e.; asterisks indicate significant differences (P = 0·05) between flooded (FL) and well-drained control plants (WD).
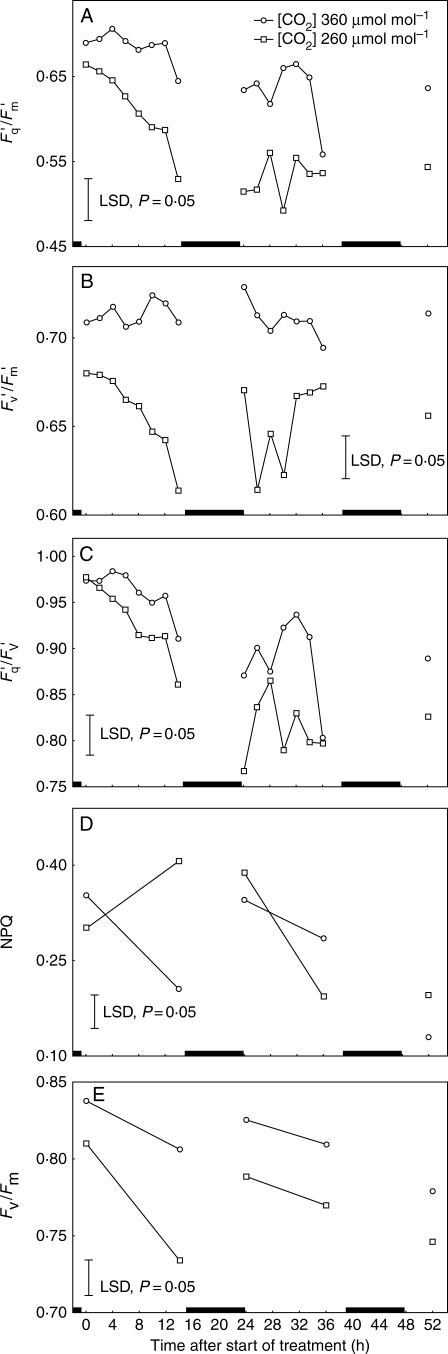
CO2 availability to well-drained plants was reduced to mimic the lowering effect of flooding and stomatal closure on Ci that was revealed by the IRGA analysis shown in Table 2. Lowering Ca by approx. 100 µmol mol−1 decreased Ci to142 µmol mol−1, which is a little below the 170–180 µmol mol−1 estimated for flooded plants after 5–28 h inundation. The lowered Ca altered fluorescence parameters in ways similar to those induced by soil flooding (Fig. 5). Fq′/Fm′, Fv′/Fm′ and Fq′/Fv′ were lessened within 8 h. During the second and third photoperiods, Fq′/Fm′, Fq′/Fv′ and Fv′/Fm′ were not reduced further. Lowering Ci increased NPQ at the end of the first photoperiod (Fig. 5D) but thereafter the effect was less clear-cut. Fv/Fm under low CO2 decreased significantly at the end of the first photoperiod and at the beginning of the second photoperiod, but did not decline further at later times.
Fig. 5.
Effect of reducing Ca by 100 µmol mol−1 on (A) quantum efficiency of PSII photochemistry (Fq′/Fm′), (B) the operating efficiency of PSII photochemistry (Fv′/Fm′), (C) photochemical fluorescence quenching (Fq′/Fv′), (D) non-photochemical fluorescence quenching (NPQ) and (E) maximum quantum efficiency of PSII photochemistry (Fv/Fm) of well-drained tomato plants. Plants were dark-adapted for at least 20 min prior to measurement of F0 and Fm to estimate NPQ and Fv/Fm. Each point represents the mean of 24 replicate measurements made on the fifth oldest leaves of eight 1-month-old plants per treatment. Vertical lines are LSDs at P = 0·05. Black boxes on the x-axis indicate dark periods.
Impact of adventitious roots on stomatal closure and chlorophyll fluorescence
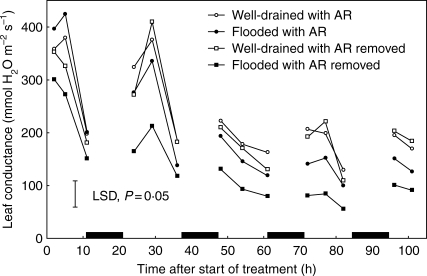
Fast elongating and much-branched adventitious roots started emerging from the hypocotyl into the upper surface of the floodwater after 3 d. The number of such axes increased from 3·5 on day 3 to 7·6 after 4 d, 13·5 after 6 d and to about 20 much-branched axes after 12 d (Table 3). During this time, leaf conductances of flooded plants (seventh oldest leaf) gradually increased. This indicated that closure was slowly being reversed by the actions of an increasingly large well-aerated root system. In association with the increasing leaf conductances, the fluorescence parameter Fq′/Fm′ was found to have recovered substantially by the end of the experiment. In a different approach, adventitious roots were induced to grow in well-aerated peat prior to the start of flooding to test their impact on stomata. In well-drained plants, excising or retaining the adventitious roots made little difference to leaf conductances over five photoperiods, except during the first day when some reduction was seen if roots were removed. However, the presence of pre-formed adventitious roots increased leaf conductances of flooded plants significantly, although values remained below those of well-drained plants (Fig. 6).
Table 3.
Effect of extending flooding of 1-month-old tomato plants to 9–10 d on adventitious root formation, leaf conductance and PSII operating quantum efficiency (Fv′/Fm′) of the seventh oldest leaf
| Days of treatment |
|||||||
|---|---|---|---|---|---|---|---|
| 3 | 4 | 5 | 6 | 7 | 8 | 9 | |
| Number of adventitious roots | |||||||
| Well-drained | 0 | 0 | 0 | 0 | |||
| Flooded | 3·5 ± 1·9 | 7·3 ± 2·4 | 13·4 ± 2·3 | >20 | |||
| Leaf conductance (mmol m−1 s−1) | |||||||
| Well-drained | 395 ± 46 | 500 ± 43 | 590 ± 36 | >600 | 700 ± 89 | 470 ± 6 | 370 ± 66 |
| Flooded | 61 ± 15 | 70 ± 7 | 90 ± 11 | 100 ± 11 | 100 ± 7 | 90 ± 10 | 110 ± 11 |
| Operating quantum efficiency of PSII (Fq′/Fm′) | |||||||
| Well-drained | 0·480 ± 0·015 | 0·461 ± 0·023* | |||||
| Flooded | 0·266 ± 0·011 | 0·406 ± 0·011* | |||||
Means with s.e. (n = 8 for stomatal conductance and n = 24 for operating quantum efficiency of PSII).
*Measured in the morning on day 10.
Fig. 6.
Effect of retaining or removing a pre-formed and well-aerated adventitious root system (AR) on leaf conductances of the third oldest leaves of flooded and well-drained 1-month-old tomato plants.
Impact of cytokinin and gibberellic acid treatment
When sufficient hormone was applied to double leaflet elongation rates in flooded plants, there was a small (30 %) and statistically non-significant increase in leaf conductance after 30 h (Table 4). A similar relative effect was seen in well-drained plants but, statistically and in absolute terms, the response by well-drained plants was much stronger than in flooded plants. Conductance measurements at earlier and later times (up to 85 h) gave similar relative values (data not shown). Flooding for approx. 30 h was sufficient to reduce Fq′/Fm′, Fq′/Fv′ and Fv/Fm, and raise NPQ. Hormone treatment had no statistically significant effect on the response of any of these parameters to 30 h flooding (Table 4) or at later times up to approx. 85 h (result not shown).
Table 4.
Effect of pre-treating the leaves of 1-month-old tomato plants with benzyladenine and gibberellic acid (10 mg L−1) on responses to flooding of the soil for approx. 30 h
| Leaf extension (mm) | Leaf conductance (mmol m−2 s−1) | Fq′/Fm′ | Fq′/Fv′ | Fv′/Fm′ | NPQ | Fv/Fm | |
|---|---|---|---|---|---|---|---|
| Well-drained | |||||||
| No hormone | 25·5 ± 2·2 | 488·9 ± 45·59 | 0·575 ± 0·013 | 0·817 ± 0·019 | 0·703 ± 0·003 | 0·162 ± 0·014 | 0·717 ± 0·004 |
| With hormone | 31·8 ± 2·0 | 631·3 ± 55·01 | 0·552 ± 0·017 | 0·792 ± 0·024 | 0·696 ± 0·004 | 0·192 ± 0·015 | 0·722 ± 0·003 |
| Flooded | |||||||
| No hormone | 15·0 ± 0·8* | 224·4 ± 21·67* | 0·512 ± 0·020* | 0·733 ± 0·029* | 0·700 ± 0·006NS | 0·199 ± 0·016NS | 0·699 ± 0·005* |
| With hormone | 30·4 ± 1·9** | 289·3 ± 33·64ns | 0·462 ± 0·023ns | 0·647 ± 0·032ns | 0·714 ± 0·005ns | 0·167 ± 0·020ns | 0·711 ± 0·004ns |
Results are means ± s.e.
*Flooded plants significantly different from well-drained plants (P ≤ 0·05).
**Flooded plants with hormone significantly different from flooded plants with no hormone (P ≤ 0·05).
NS, not statistically different from well-drained plants. ns, not statistically different from flooded plants with no hormone.
Leaf extension by the terminal leaflet of the youngest expanding leaf was measured over the first 47 h of flooding (n = 8). Leaf conductances were from the fifth oldest leaf (n = 8) after 27 h flooding. Fluorescence parameters were from the fifth oldest leaf after approx. 30 h flooding (n = 24 readings from eight plants).
DISCUSSION
Background
One or more signals generated directly or indirectly by roots are presumed to be responsible for initiating stomatal closure in flooded plants (Jackson, 2002). In pea (Pisum sativum), an accumulation message in the form of ABA accretion in leaves due to inhibited export to roots seems to be the signal (Jackson and Hall, 1987) while in Ricinus communis, a negative message in the form of severe leaf water deficit generated by loss of root hydraulic conductivity is able to induce closure (Else et al., 2001). However, in flooded tomato plants, the active signals have remained elusive. One possibility, now largely rejected, is that early closure is a response to increased output of ABA from roots, as reported for drying roots (e.g. Jokhan et al., 1996). This possibility was convincingly ruled out by the finding that ABA delivery to the leaves via xylem sap is strongly reduced rather than increased by >2–4 h flooding (Else et al., 1996). Also largely discounted has been ethylene derived from its precursor 1-aminocyclopropane-1-carboxylic acid (ACC) that passes in increased amounts from flooded roots to the shoot system in xylem sap (Bradford and Hsaio, 1982; Else and Jackson, 1998). However, a recent description of an ethylene-responsive transcription factor (ERF1) that is ABA inducible (Xu et al. 2007) suggests that this possibility should be re-investigated since ABA concentrations can increase to a small extent in leaves of flooded tomato plants, probably as a consequence of impeded export (Else et al., 1996) and internal redistribution (Else et al. 2006). This ABA may conceivably close stomata by interacting with extra ethylene derived from ACC delivered in xylem sap. Other potential signals closing stomata include a rapid increase in xylem sap pH and an equally prompt reduction in the delivery of nitrate and other anions to shoots. However, while these may be effective signals in plants with roots in drying soil (Radin et al. 1982; Wilkinson and Davies 1997), direct tests with tomato proved negative (Jackson et al. 2003).
Flooding, leaf water potentials and stomatal closure
As shown in Fig 1B and C, prompt stomatal closure is intimately linked to increased leaf water potentials (ψL) and slower transpiration (Fig. 2). This confirms the adaptive role of stomatal closure in counteracting loss of leaf hydration caused by smaller root hydraulic conductivities of newly flooded roots. However, the start of stomatal closure may sometimes be too late to prevent an early, albeit temporary, loss of ψL and an associated loss of leaf hydration. This could conceivably trigger the subsequent closing of stomata. However, such a hypothesis was not supported by the present experiments. When flooding commenced at the start of the photoperiod, stomatal closure was indeed preceded by a short period of more negative ψL, suggesting a causal link. However, when flooding was started at the end of the photoperiod, the pattern of stomatal closure in the subsequent photoperiod remained unaffected despite the absence of a preceding period of lower ψL (Fig. 1A). This finding supports other experimental work (Else et al., 1995) indicating that hydraulic signalling of this kind is not necessarily responsible for closing stomata in flooded tomato plants.
Photosynthesis and stomatal closure in flooded plants
One aim of the present work was to investigate the possibility that increases in Ci, brought about by loss of function of PSII, may close the stomata. The notion is supported by two reports of Ci increasing in the leaves of flooded plants (Guidi and Soldatini, 1997; Yordanova and Popova, 2007) and of damage to light-harvesting mechanisms in flooded tomato (Janowiak et al., 2002) and soybean (Ahmed et al., 2006). CO2 enrichment might also come about because of increased delivery to leaves as dissolved gas in the transpiration stream (Saveyn et al., 2008) originating in the flooded soil where considerable quantities of CO2 accumulate within 1 h (Else et al., 1995). Other possibilities include increased photorespiration and reduced ribulose bisphosphate (RuBP) activity (Pezeshki, 1994; Yordanova and Popova, 2007) arising from reduced capacity to regenerate (Bradford, 1983b) in the face of sharp decreases in nitrate supply from roots (Jackson et al., 2003) or slowed rates of repair (Nishiyama et al., 2006). A recent proteomics study using MALDI-TOF (matrix-assisted laser desorption ionization) and western blotting revealed a significant degradation of RuBP and RuBP activase in the leaves of 5-week-old tomato plants after 3 d soil waterlogging (Ahsan et al, 2007), seemingly the outcome of oxidative damage to membranes by H2O2. The possibility that increases in Ci close the stomata of flooded plants was backed up further by the present finding that modest increases in Ci brought about by raising external concentrations by 100 µmol mol−1 strongly slowed transpiration, a marker of stomatal closure (Table 1).
Fluorescence analysis was then used to examine the functionality of PSII in combination with conventional gas exchange measurements. In these ways, a sequence of flooding-induced changes in chlorophyll a fluorescence and leaf gas exchange was established during the first few days of flooding. Three florescence parameters were decreased substantially in flooded plants during the first day of flooding (Fig. 3A and C; Table 3). The depressed parameters were: Fq′/Fm′ (operating quantum efficiency of PSII and a measure of the proportion of absorbed light used in carbon fixation); Fv′/Fm′ (a second measure of the operating quantum efficiency of PSII photochemistry and of fluorescence yield from electron transfer in PSII in bright light) and Fq′/Fv′ (a measure of photosynthetic photochemical fluorescence quenching and an indicator of the capacity for photochemistry by PSII). Decreases in Fv/Fm, an estimate of the maximum quantum yield of PSII and an indicator of damage to reaction centres of PSII, emerged somewhat later (Fig. 3E). At no time did changes to fluorescence parameters precede stomatal closure, while the late onset of Fv/Fm decline suggests that any actual damage to PSII took place some considerable time after stomata closed. If early PSII inhibition closed stomata by raising Ci, conventional gas exchange analysis should detect the increase. However, IRGA analysis showed Ci to decrease rather than increase as stomata closed (Table 1). This decrease is the likely cause of an observed depression of CO2 assimilation (A and Amax), although some loss of carboxylation efficiency (the responsiveness of assimilation to increases in CO2) may also contribute during the second photoperiod (Table 2). The decreased carboxylation efficiency, and also a rise in the CO2 compensation point (Table 2), implies some damage to PSII or to downstream enzymic steps in CO2 fixation. However, overall, these findings give little support to the hypothesis that early damage to PSII and an associated increase in Ci explains the prompt closing of stomata by flooded tomato plants.
Changes in fluorescence parameters result from stomatal closure
Instead of losses in PSII efficiency initiating stomatal closure, the closure seems more likely to cause losses in PSII efficiency, probably by lessening of CO2 availability for photosynthesis. The direct measurements of Ci (Table 2) showed decreases commencing 5–6 h after the start of flooding and in synchrony with declining stomatal apertures (lower gs). Furthermore, stomatal closure reduced Fq′/Fm′ within 1 h when closure was induced in detached leaves by supplying ABA (see text in Results for data). Also, when CO2 shortage similar in extent to that experienced by flooded plants was imposed on well-drained plants by reducing Ca, this altered fluorescence parameters in similar ways to flooding although there were temporal differences (compare Figs 3 and 5) possibly because reduction of Ci was greater in the flooded plants. Lawson et al. (2002) also reported a decrease in Fq′/Fm′ when leaves (Commelina communis) were starved of CO2. The limited availability of CO2 resulting from stomatal closure would be likely to depress the amounts of electron-accepting NADP+ as the carbon reduction cycle slowed. Any resulting inhibition of photochemical utilization of incident light would then depress values for Fq′/Fm′, and also Fv′/Fm′ and Fq′/Fv′. Close correlation between the extent of stomatal closure and decreases in Fq′/Fm′ (Fig. 4A) over 3 d of flooding emphasizes the likely causal link.
Flooding also decreased values of Fv/Fm, a parameter often associated with unrepaired damage to PSII rather than just with reduced operating efficiency. The decreases in Fv/Fm developed more slowly and less strongly than those for Fq′/Fm′ (and Fv′/Fm′ and Fq′/Fv′) during flooding and thus correlated much less closely with stomatal closure (Fig. 4). Since depriving well-drained plants of CO2 also depressed Fv/Fm to some extent (Fig. 5E), there may be no need to turn beyond CO2 shortage for the initial cause in flooded plants. A conventional explanation would be that with less CO2 available for photosynthesis, surplus reducing power is diverted to O2 and the generation of potentially damaging superoxide anions (O−2) and H2O2 (Yan et al., 1996; Yordanova and Popova, 2007). The effect appears to be too strong to be overcome by increases in NPQ that both flooding and increased Ci bring about (Figs 3D and 5D). Increases in NPQ are thought to reflect an adaptive diversion of light energy and thus of reductive power away from PSII. It is concluded that the changes in photosynthetic fluorescence parameters resulting from up to 3 d of flooding are the result of stomatal closure rather than its cause and are induced mainly by restricted availability of CO2 for photosynthetic reduction. Other factors damaging PSII during prolonged flooding cannot be ruled out and may explain the larger effects of flooding on Fv/Fm loss compared with the effects of CO2 deprivation alone.
Impact of adventitious roots, cytokinin and gibberellin
Delivery of cytokinin and gibberellin from roots to shoots in the transpiration stream is strongly reduced by flooding, based on the bioassay evidence of decreased xylem sap concentrations published by Burrows and Carr (1969) and Reid et al. (1969). Gibberellins (Kumar et al., 2004) and, in particular, cytokinins may promote stomatal opening and antagonize the closing tendencies of ABA (Pospíšilová, 2003). The ability of pre-formed well-aerated roots to overcome stomatal closing induced by flooding the main root system was therefore assessed. Retaining a well-aerated root system above the flooded roots succeeded in partially overcoming stomatal closing over at least 4 d of flooding (Fig. 6). By implication, the effect could be a consequence of the cytokinins and gibberellins exported to the leaves. However, the offsetting effect of well-aerated roots appears to have another explanation since the effect could not be reproduced by pre-treating flooded plants with a combined foliar spray of the aromatic cytokinin BA and GA (Table 4) even though the amounts given were enough to stimulate leaf elongation. One possible explanation for the modest opening influence of adventitious roots is that as they develop they supply an increasing proportion of the total transpiration flow. This would suppress the export of any stomatal-closing factors from the stressed root system in xylem sap if water flow rather than transmembrane diffusion or active transport were driving entry of solutes and a putative promoter of stomatal closure into xylem sap. There was also no effect of hormone application on the five chlorophyll fluorescence parameters (Table 4). This is in line with the notion that flood-induced changes in chlorophyll fluorescence arise mainly from stomatal closure and not directly from a loss of root signals such as cytokinin and gibberellin. In a second approach, adventitious roots were allowed to emerge naturally from the hypocotyl into the well-aerated flood water above the soil surface. Over 3, 6 and 9 d of flooding this new root system grew to a substantial size (Table 3). This resulted in a small and gradual increase in leaf conductance culminating in a recovery of Fq′/Fm′, indicating that well-aerated roots can counteract the effect of flooded roots on stomatal behaviour and dependent fluorescence parameters.
CONCLUSIONS
No evidence was found that stomatal closure following soil flooding is initiated by short-term loss of leaf hydration. Similarly, no evidence was found that loss of PSII efficiency and associated increases in internal CO2 were responsible for the closure. On the contrary, early loss of PSII functionality and later damage were found to be mostly the consequences of stomatal closure and the resulting lower internal CO2. Well-aerated roots induced either before or during flooding restored stomatal apertures to some extent. This suggests that the signal(s) for closure could be negative messages in the form of a loss of factors from roots such as cytokinins and gibberellins that normally promote opening. However, the failure of exogenous cytokinin and gibberellin to mimic the effect of well-aerated roots indicates that the active negative messages are unlikely to include reduced supplies of native hormones of this type, although more work on this question is needed. An alternative explanation is that transpiration flow drawn through the flooded roots is partially replaced by that through well-aerated adventitious roots, thereby sweeping less promoter out of flooded roots and into the leaves. This could reduce delivery of an as yet unidentified stomatal-closing factor from the oxygen-deficient root system (Else et al., 1996, 2006), but only if water flow rate is the driving force behind its entry into xylem sap of the flooded roots. Recent evidence of positive interactions between ethylene and ABA (Xu et al., 2007; but see Tanaka et al., 2006), and earlier work indicating that ABA is needed for stomata to close in flooded plants known to be enriched with ethylene (Jackson and Hall, 1987) indicates that stomatal closure through an ABA–ethylene interaction cannot be ruled out.
ACKNOWLEDGEMENTS
We thank Ms Kazimiera Hajduk and Mr Marek Trybala for technical assistance, Dr Tracy Lawson for helpful discussions, and Professor David Dunstan for comments on an earlier version of the manuscript. Financial support from the Royal Society of London is gratefully acknowledged.
LITERATURE CITED
- Ahmed S, Nawata E, Sakuratania T. Changes of endogenous ABA and ACC, and their correlations to photosynthesis and water relations in mungbean (Vigna radiata (L.) Wilczak cv. KPS1) during waterlogging. Environmental and Experimental Botany. 2006;57:278–284. [Google Scholar]
- Ahsan N, Lee D-G, Lee S-H, Kang K Y, Bahk J D, Choi M S, et al. A comparative proteomic analysis of tomato leaves in response to waterlogging stress. Physiologia Plantarum. 2007;131:555–570. doi: 10.1111/j.1399-3054.2007.00980.x. [DOI] [PubMed] [Google Scholar]
- Baker NR, Oxborough K, Lawson T, Morrison JIL. High resolution imaging of photosynthetic activities of tissues, cells and chloroplasts in leaves. Journal of Experimental Botany. 2001;52:615–621. [PubMed] [Google Scholar]
- Bradford KJ. Involvement of plant growth substances in the alteration of leaf gas exchange of flooded tomato plants. Plant Physiology. 1983;a 73:480–483. doi: 10.1104/pp.73.2.480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford KJ. Effects of soil flooding on leaf gas exchange of tomato plants. Plant Physiology. 1983;b 73:475–479. doi: 10.1104/pp.73.2.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford KJ, Hsaio TC. Stomatal behaviour and water relations of waterlogged tomato plants. Plant Physiology. 1982;70:1508–1513. doi: 10.1104/pp.70.5.1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burrows WJ, Carr DJ. Effects of flooding the root system of sunflower on the cytokinin content in the xylem sap. Physiologia Plantarum. 1969;22:1105–1112. doi: 10.1111/j.1399-3054.1969.tb09098.x. [DOI] [PubMed] [Google Scholar]
- Else M.A, Jackson MB. Transport of 1-aminocyclopropane-1-carboxylic acid (ACC) in the transpiration stream of tomato (Lycopersicon esculentum) in relation to foliar ethylene production and petiole epinasty. Australian Journal of Plant Physiology. 1998;25:453–458. [Google Scholar]
- Else MA, Davies WJ, Malone M, Jackson MB. A negative hydraulic message from oxygen-deficient roots of tomato plants? Influence of soil flooding on leaf water potential, leaf expansion, and synchrony between stomatal conductance and root hydraulic conductivity. Plant Physiology. 1995;109:1017–1024. doi: 10.1104/pp.109.3.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Else MA, Tiekstra AE, Croker SJ, Davies WJ, Jackson MB. Stomatal closure in flooded tomato plants involves abscisic acid and a chemically unidentified anti-transpirant in xylem sap. Plant Physiology. 1996;112:239–247. doi: 10.1104/pp.112.1.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Else MA, Coupland D, Dutton L, Jackson MB. Hydraulic and chemical signalling in flooded and well-drained Castor oil (Ricinus communis L.) plants. Physiologia Plantarum. 2001;111:46–54. [Google Scholar]
- Else MA, Taylor JM, Atkinson CJ. Anti-transpirant activity in xylem sap from flooded tomato (Lycopersicon esculentum Mill.) plants is not due to pH-mediated redistributions of root- or shoot-sourced ABA. Journal of Experimental Botany. 2006;57:3349–3357. doi: 10.1093/jxb/erl099. [DOI] [PubMed] [Google Scholar]
- Guidi L, Soldatini GF. Chlorophyll fluorescence and gas exchanges in flooded soybean and sunflower plants. Plant Physiology and Biochemistry. 1997;35:713–717. [Google Scholar]
- Jackson MB. Long distance signalling from roots to shoots assessed: the flooding story. Journal of Experimental Botany. 2002;53:175–181. doi: 10.1093/jexbot/53.367.175. [DOI] [PubMed] [Google Scholar]
- Jackson MB. The impact of flooding stress on plants and crops (PlantStress: a website addressing plant environmental stress issues in agriculture, plant physiology, breeding, genetics and biotechnology. 2004. http://www.plantstress.com/Articles/index.asp. )
- Jackson MB. Plant survival in wet environments: resilience and escape mediated by shoot systems. In: Bobbink R, Beltman B, Verhoeven JTA, Whigham DE, editors. Wetlands: functioning, biodiversity, conservation, and restoration. Ecological Studies Vol. 191. Berlin: Springer-Verlag; 2006. pp. 15–36. [Google Scholar]
- Jackson MB, Campbell DJ. Effects of benzyl adenine and gibberellic acid on the responses of tomato plants to anaerobic root environments and to ethylene. New Phytologist. 1979;82:331–340. [Google Scholar]
- Jackson MB, Hall KC. Early stomatal closure in waterlogged pea plants is mediated by abscisic acid in the absence of foliar water deficits. Plant, Cell and Environment. 1987;10:121–130. [Google Scholar]
- Jackson MB, Saker LR, Crisp CM, Else MA, Janowiak F. Ionic and pH signalling from roots to shoots of flooded tomato plants in relation to stomatal closure. Plant and Soil. 2003;253:103–113. [Google Scholar]
- Janowiak F, Else MA, Jackson MB. A loss of photosynthetic efficiency does not explain stomatal closure in flooded tomato plants. Advances of Agricultural Sciences – Problem Issues (Warsaw) 2002;481:229–234. [Google Scholar]
- Jokhan AD, Else MA, Jackson MB. Delivery rates of abscisic acid in xylem sap of Ricinus communis L. plants subjected to part-drying of the soil. Journal of Experimental Botany. 1996;47:1595–1599. [Google Scholar]
- Kramer PJ. London: McGraw Hill; 1969. Plant and soil water relationships: a modern synthesis. [Google Scholar]
- Kumar B, Pandey DM, Goswami CL, Jain S. Effect of growth regulators on photosynthesis, transpiration and related parameters in water stressed cotton. Biologia Plantarum. 2004;44:475–478. [Google Scholar]
- Lawson T, Oxborough K, Morison JIL, Baker NR. Responses of photosynthetic electron transport in stomatal guard cells and mesophyll cells in intact leaves to light, CO2 and humidity. Plant Physiology. 2002;128:52–62. [PMC free article] [PubMed] [Google Scholar]
- Moldau H. Effects of various water regimes on stomatal and mesophyll conductances of bean leaves. Photosynthetica. 1973;7:1–7. [Google Scholar]
- Nishiyama Y, Allakhverdiev S I, Murata N. A new paradigm for the action of reactive oxygen species in the photoinhibition of photosystem II. Biochimica et Biophysica Acta. 2006;1757:742–749. doi: 10.1016/j.bbabio.2006.05.013. [DOI] [PubMed] [Google Scholar]
- Mott KA. Do stomata respond to CO2 concentrations other than intercellular? Plant Physiology. 1988;86:200–203. doi: 10.1104/pp.86.1.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oxborough K, Baker NA. Resolving chlorophyll a fluorescence images of photosynthetic efficiency into photochemical and non-photochemical components – calculation of qP and Fv′/Fm′ without measuring F0′. Photosynthesis Research. 1997;54:135–142. [Google Scholar]
- Pezeshki SR. Responses of baldcypress (Taxodium distichum) seedlings to hypoxia: leaf protein content, ribulose-1,5-bisphosphate carboxylase/oxygenase activity and photosynthesis. Photosynthetica. 1994;30:59–68. [Google Scholar]
- Pospíšilová J. Participation of phytohormones in the stomatal regulation of gas exchange during water stress. Biologia Plantarum. 2003;46:491–506. [Google Scholar]
- Radin JW, Parker LL, Guinn G. Water relations of cotton plants under nitrogen deficiency. V. Environmental control of abscisic acid accumulation and stomatal sensitivity to abscisic acid. Plant Physiology. 1982;70:1066–1070. doi: 10.1104/pp.70.4.1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid DM, Crozier A, Harvey BMR. The effects of flooding on the export of gibberellins from the root to the shoot. Planta. 1969;89:376–379. doi: 10.1007/BF00387239. [DOI] [PubMed] [Google Scholar]
- Saveyn A, Steppe K, McGuire MA, Lemeur R, Teskey RO. Stem respiration and carbon dioxide efflux of young Populus deltoides trees in relation to temperature and xylem carbon dioxide concentration. Oecologia. 2008;154:637–649. doi: 10.1007/s00442-007-0868-y. [DOI] [PubMed] [Google Scholar]
- Schildwacht PM. Is a decreased water potential after withholding oxygen from the roots the cause of the decline of leaf-elongation rates in Zea mays L. and Phaseolus vulgaris L.? Planta. 1989;177:178–184. doi: 10.1007/BF00392806. [DOI] [PubMed] [Google Scholar]
- Tanaka Y, Sano T, Tamaoki M, Nakajima N, Kondo N. Cytokinin and auxin inhibit abscisic acid-induced stomatal closure by enhancing ethylene production in Arabidopsis. Journal of Experimental Botany. 2006;57:2259–2266. doi: 10.1093/jxb/erj193. [DOI] [PubMed] [Google Scholar]
- Tournaire-Roux C, Sutka M, Javot H, Gout E, Gerbeau P, Luu DT, Bligny R, Maurel C. Cytosolic pH regulates root water transport during anoxic stress through gating of aquaporins. Nature. 2003;425:393–397. doi: 10.1038/nature01853. [DOI] [PubMed] [Google Scholar]
- Wadman-van-Schravendijk H, van Andel OM. Interdependence of growth, water relations, and abscisic acid level in Phaseolus vulgaris during waterlogging. Physiologia Plantarum. 1985;63:215–220. [Google Scholar]
- Walz H. Portable Fluorometer PAM-2000 and data acquisition software DA-2000. Handbook. 2nd edn. Germany: Effeltrich; 1993. [Google Scholar]
- Wilkinson S, Davies WJ. Xylem sap pH increase. A drought signal received at the apoplastic face of the guard cell that involves the suppression of saturable abscisic acid uptake by the epidermal symplast. Plant Physiology. 1997;113:559–573. doi: 10.1104/pp.113.2.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Z-S, Xia L-Q, Chen M, Cheng X-G, Zhang R-Y, Li L-C, et al. Isolation and molecular characterization of the Triticum aestivum L. ethylene-responsive factor 1 (TaERF1) that increases multiple stress tolerance. Plant Molecular Biology. 2007;65:719–732. doi: 10.1007/s11103-007-9237-9. [DOI] [PubMed] [Google Scholar]
- Yan B, Dai Q, Liu X, Huang S, Wang Z. Flooding-induced membrane damage, lipid oxidation and activated oxygen generation in corn leaves. Plant and Soil. 1996;179:261–268. [Google Scholar]
- Yordanova RY, Popova LP. Flooding-induced changes in photosynthesis and oxidative status in maize plants. Acta Physiologiae Plantarum. 2007;29:535–541. [Google Scholar]