Figure 1. BimEL does not undergo tyrosine phosphorylation under conditions known to target it for degradation.
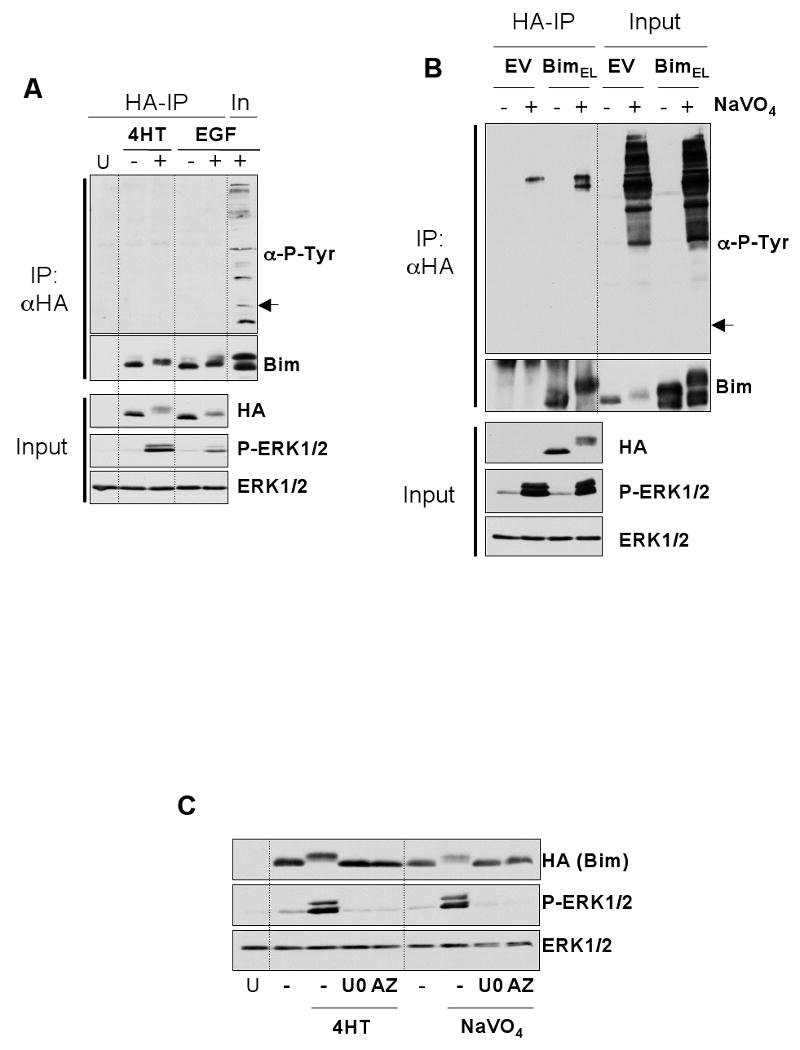
HR1 cells were transfected with plasmids encoding HA-BimEL or empty vector. After 16 hours, cells were serum starved for 30 minutes and then stimulated with 100nM 4-hydroxytamoxifen (4-HT) for 1 hour or 10nM EGF for 5 minutes (A) or pervanadate (see materials and methods) for 10 minutes (B). Whole cell extracts were then prepared, normalised for protein content, and boiled directly (input) or used for immunoprecipitation of HA-BimEL. Input and IP samples were then immunoblotted with antibodies to anti-phosphotyrosine, Bim, HA (Bim), P-ERK1/2 and ERK1/2. Input lysates were included with the IP samples to act as a positive control for the anti-phosphotyrosine immunoblot. Note: the arrow in (A) & (B) indicates the position at which Bim resolves and would appear if it reacted with the anti-P-Tyr antibody. (C) Phosphorylation of BimEL after treatment with 4-HT or pervanadate is ERK1/2 dependent. HR1 cells were transfected with HA-BimEL for 16 hours, then switched to serum free medium ± 20μM UO126 or 10μM AZD6244. Cells were stimulated with 100nM 4-HT for 1 hour or pervanadate for 10 minutes (see materials and methods) and whole cell extracts prepared for immunoblot analysis using antibodies to HA (Bim), P-ERK1/2 and ERK1/2. Results are from single representative experiments; similar results were obtained in a total of three experiments.
