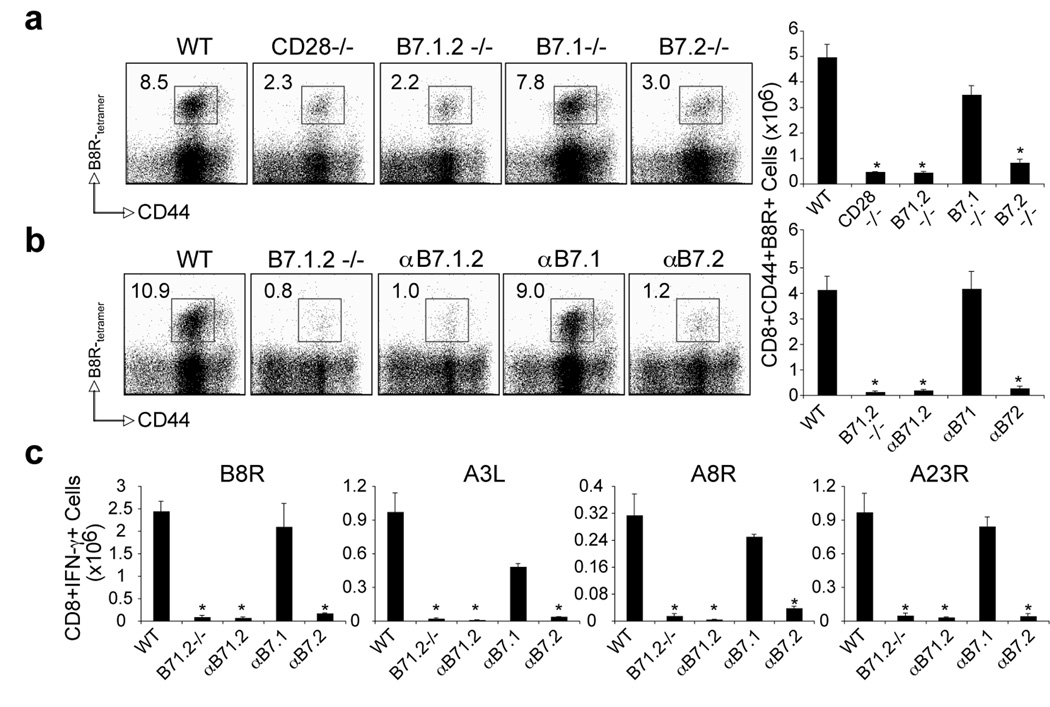
Figure 3. Differential requirement for B7.1 and B7.2 in primary VACV-specific CD8 T cell responses to both dominant and subdominant VACV epitopes.
(a) WT, CD28−/−, B7.1/B7.2-double deficient (B7.1.2−/−), B7.1−/−, or B7.2−/− mice were infected i.p with VACV-WR (2 × 105 PFU/mouse). Seven days post-infection splenocytes were harvested and stained for CD8, CD44, and B8R-tetramer. Left: Representative plots of tetramer staining, gating on CD8 cells. Percentages of activated B8R-tetramer positive CD8 T cells (CD8+CD44+B8R+) are indicated. Right: Total numbers of B8R-tetramer positive CD8+CD44+ T cells per spleen. (b). WT mice were infected as (a). Groups of mice were injected i.p on days 0, 1, 2, and 3, with 150 µg of either control IgG or non-depleting anti-B7.1, anti-B7.2, or anti-B7.1 plus B7.2 blocking antibodies in PBS. Groups of B7.1.2−/− mice were used as positive control. At day 7 post-infection splenocytes were harvested and stained for CD8, CD44, B8R-tetramer. Left: Representative plots of B8R-tetramer staining, gating on CD8 T cells. Right: Total number of CD8+CD44+B8R+ T cells per spleen is shown. (c) At day 7, splenocytes from wt and CD28−/− infected mice were stimulated with B8R, A3L, A3R, and A23R peptides for intracellular IFN-γ staining. Total numbers ± SEM of CD8+IFN-γ+ T cells per spleen from four individual mice are shown. *, p < 0.05 (WT vs knockout or blocking Ab treated groups). Similar results were obtained in 3 separate experiments.