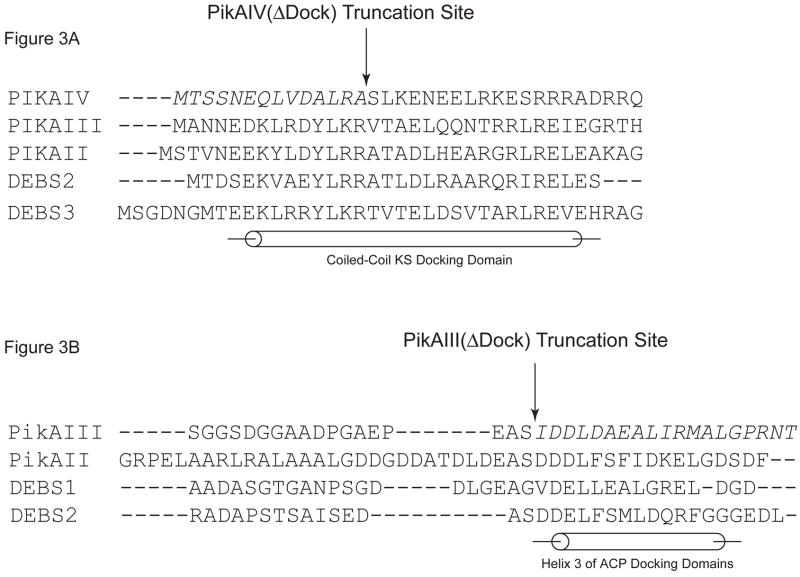
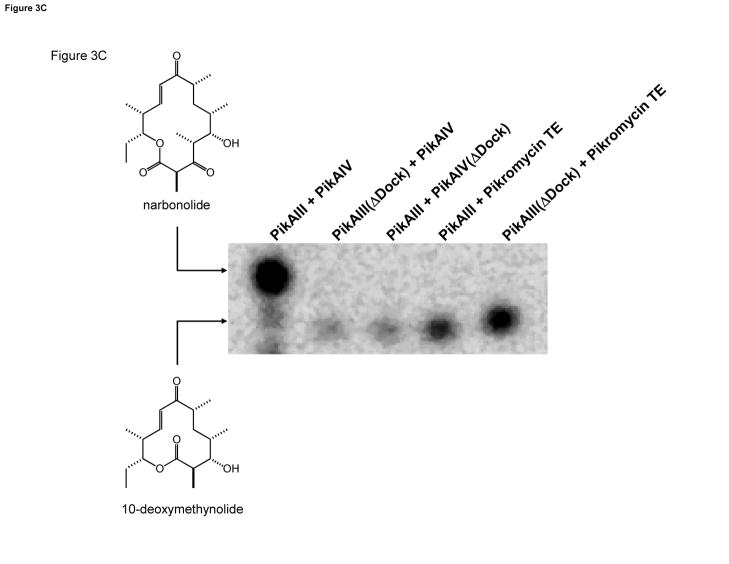
Figure 3. PikAIII(ΔDock) and PikAIV(ΔDock): Design and Activity.
(A). Multiple sequence alignment of the helical N-terminal docking domains from the pikromycin and DEBS PKS. PikAIV(ΔDock) is the result of truncating the first 14 amino acids (in italics) of PikAIV. (B). Multiple sequence alignment of partial C-terminal docking domains from the pikromycin and DEBS PKS. PikAIII(ΔDock) is the result of truncating the final 19 amino acids (in italics) of PikAIII that comprise helix 3. (C). Radio-TLC of reaction products produced by the truncated pikromcyin modules. PikAIII(ΔDock) or PikAIV(ΔDock) was paired with their respective partner wild-type or truncated module in the presence of the synthetic pentaketide NAC thioester and 2-[14C]-methylmalonyl-CoA as described in the experimental procedures. Likewise, PikAIII or PikAIII(ΔDock) was paired with recombinant pikromycin TE under identical experimental condtions. The reaction products were resolved by TLC (5% MeOH in CHCl3) and subsequently visualized using a Typhoon phosphorimager.