Figure 3.
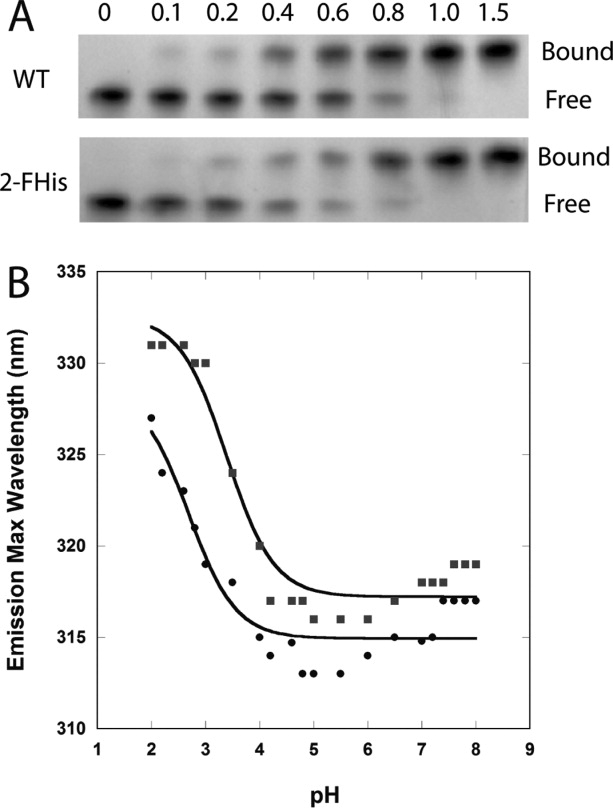
(A) Binding assay of WT PA with varying molar ratios of WT-CMG2 (top) and 2-FHis-CMG2 (bottom). Free refers to free PA and bound refers to the PA—CMG2 complex. (B) WT CMG2 (▪) and 2-FHis-CMG2 (•) pH dependence on fluorescence (Ex. 280 nm) was monitored by following the peak maximum wavelength. Data were acquired on a Cary Eclipse fluorescence instrument, and samples maintained at 20°C with a Peltier cooling system. Protein concentration was 0.8 μM, and the buffer consisted of 10 mM each of BisTris/Hepes/cacodylic acid/citric acid. The data were fit according to the Henderson-Hasselbalch equation assuming a two-state protonation equilbrium: Fl(obs) = (Fl(N) + Fl(I) 10pH-pKapp)/(1 + 10pH-pKap) where pKapp represents an apparent pKa encompassing all classes of titratable sites. The fits (solid lines) gave a pKapp of 3.4 ± 0.1 for the WT protein and 2.7 ± 0.2 for the 2-FHis labeled protein.
