Figure 1.
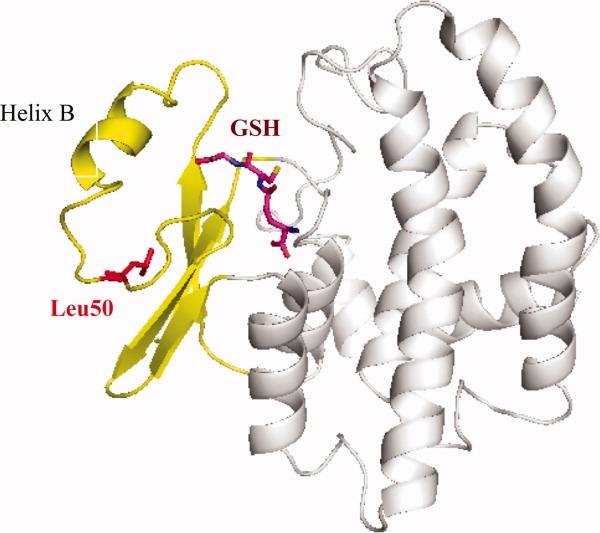
Ribbon representation of the overall structure of wild-type GST in the presence of glutathione (GSH, shown as stick model in purple). The smaller amino terminal domain of the protein is colored in yellow with Leu50 highlighted in red. This residue is pointing toward the hydrophobic core of this smaller domain of the protein. It is located at ∼10 Å distance from the substrate binding site. Any pH-sensitive effects of the Asp50, Cys50, or the His50 mutants, therefore, must be transmitted through conformational changes to the binding site. This figure as well as Figures 4 and 5 were prepared with the program PyMol (DeLano Scientific LLC, http://pymol.sourceforge.net).
