Figure 1.
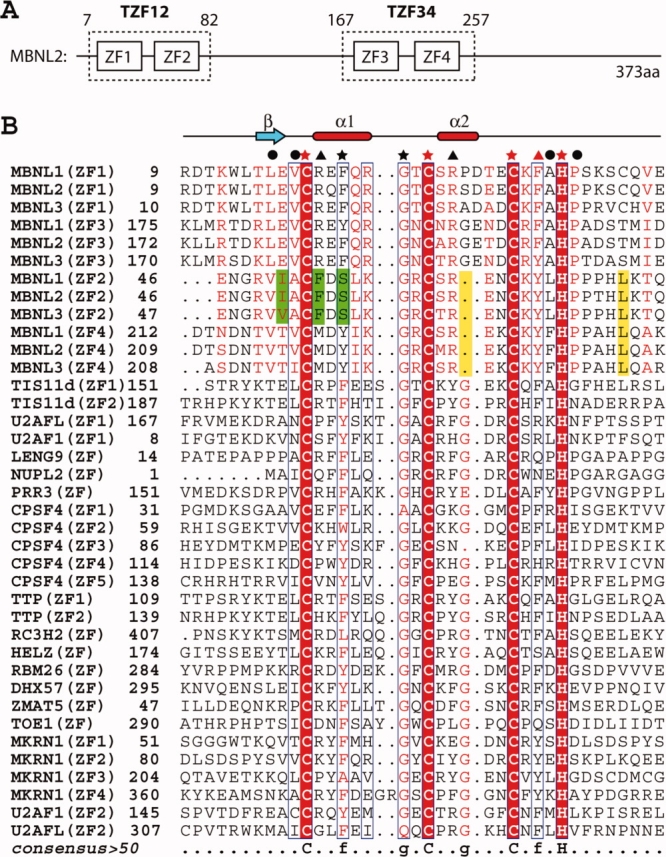
(A) Schematic diagram of the CCCH-type zinc finger motifs contained in human MBNL2. (B) Multiple sequence alignment of CCCH-type zinc finger motifs from selected human proteins. The three Cys and one His ligand residues involved in zinc coordination are marked by red stars. Other strongly conserved residues in CCCH-type zinc fingers are marked by black stars. Residues involved in hydrophobic interactions within the TZF domains are marked by black circles. Residues under the black and red triangles are the amino acid residues with different (aromatic and positively charged residues) and the same properties as compared with the residues at the corresponding positions of the CCCH zinc finger in TIS11d, respectively. Characteristic residues or positions from ZF2 only and from ZF2 and ZF4 are colored green and yellow, respectively. Secondary structure elements are depicted above the aligned sequences. The sequence alignment output was created with the ESPript program.13 [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
