Figure 3.
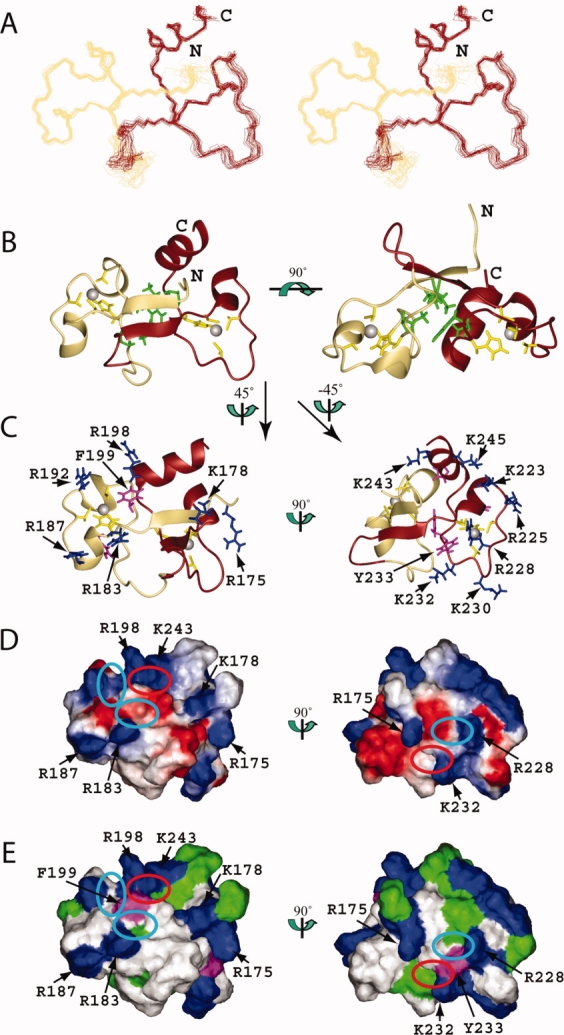
Solution structure of the TZF34 domain in human MBNL2. (A) Stereo-view of the best 20 structures of the TZF34 domain (residues Leu174-Ala247). ZF3 and ZF4 of TZF34 are colored khaki and brown, respectively. (B) Ribbon presentation of the lowest energy structure of the TZF34 domain. The colors for the backbones of ZF3 and ZF4 are the same as in A. The side chains of the ligand residues and the zinc ions are shown in yellow and grey, respectively. The side chains of the hydrophobic residues that constitute the hydrophobic core of TZF34 are shown in green. The ribbon diagram in the right panel is rotated horizontally by 90° from that on the left. (C) Ribbon diagrams of TZF34 with the side chains of the positively charged and aromatic residues of ZF3 (left panel) and ZF4 (right panel), which are shown in blue and magenta, respectively. (D) Electrostatic surface potential of the TZF34 domain. The blue and red colors represent positive and negative electrostatic surface potential, respectively. (E) Hydrophobic and positively charged surface residues of the TZF34 domain. In C, D, and E, the ZF3 and ZF4 surface orientations (left and right panel) were obtained by 45° and −45° vertical rotations of the left ribbon structure on the left in B. The ribbon diagrams (C), the electrostatic potential (D) and the hydrophobic surfaces (E) have the same orientation. The cyan and red ellipses on the surfaces (D, E) of TZF34 mark the pockets formed by the conserved residues. The red ellipse marks the pocket that results from the formation of a compact global fold with the tandem zinc fingers. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
