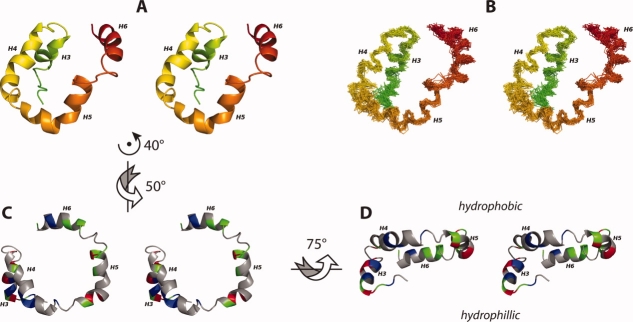
Figure 5.
The EM domain of Rv1761c is characterized by a set of four helices (A, B). These four helices are organized such that H4 and H5 are co-planar and both H3 and H6 are behind the H4/H5 plane (A, B). The helical domain is shown as a ribbon model (A) and a backbone trace for the 30 lowest energy structures (B) colored in a rainbow (as in Fig. 3A, B). The 30 superimposed backbone structures (B) are aligned to the average backbone structure using NH, Cα, and C′ atoms from helices H3, H4, H5, and H6 (Residues 59–67, 73–86, 90–103, and 110–120). The U-shaped H4/H5 helical pair forms a hydrophobic surface (C) with the amphipathic Helix H6 situated just below the H4/H5 plane and the hydrophilic Helix H3 located well below the H4/H5 plane (D). For C, D positively charged residues are colored red (Arg, His, Lys), negatively charged residues are colored blue (Asp, Glu), nonpolar residues are colored grey (Ala, Gly, Ile, Leu, Met, Pro, Phe, Ser, Thr, Val) and polar uncharged residues are colored green (Asn, Cys, Gln, Trp, Tyr). Throughout this figure, the flexible carboxyl terminal region (residues 122–127) and the flexible linker region between the TM and H3 are not shown. Arrows and axes indicate the approximate rotations used to transform between the orientations depicted in A, C, and D. Figure generated using PyMOL.44.