Figure 1.
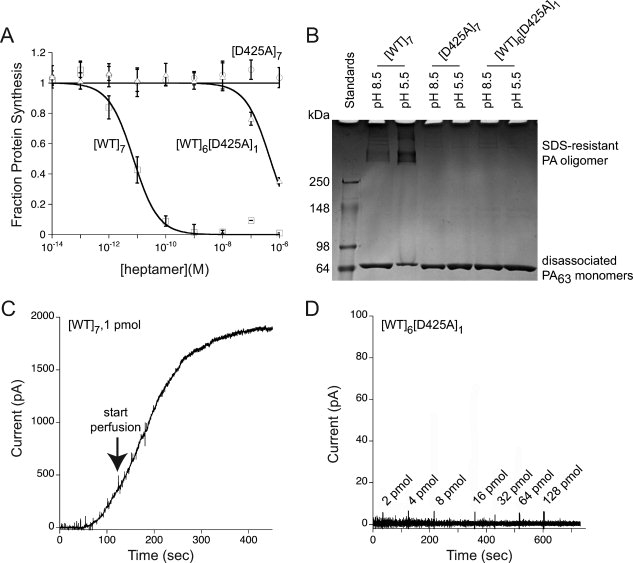
Effect of a single-subunit D425 mutation in the PA prepore. (A) Comparison of [WT]6[D425A]1 and [D425A]7 with [WT]7 in mediating entry of LFN-DTA into CHO-K1 cells. Cells were incubated with [WT]7, [WT]6[D425A]1, and [D425A]7 at indicated concentrations in the presence of 100 nM LFN-DTA. After 16 h, we measured the incorporation of 3H-leucine into protein over a 1-h period. The data plotted are averages of three experiments, with standard error shown. (B) Comparison of [WT]6[D425A]1 and [D425A]7 with [WT]7 in forming a SDS-resistant oligomer under acidic conditions. Lane 1, molecular weight markers; Lane 2, [WT]7, pH 8.5; Lane 3, [WT]7, pH 5.5; Lane 4, [D425A]7, pH 8.5; Lane 5, [D425A]7, pH 5.5; Lane 6, [WT]6[D425A]6, pH 8.5; Lane 7, [WT]6[D425A]1, pH 5.5. Each lane contains ∼3 μg prepore PA, and the gel was stained with Coomassie Brilliant Blue. (C) Monitoring of pore insertion by macroscopic current over time of 1 pmol [WT]7 upon addition to planar lipid bilayers (voltage +20 mV, pH 5.5). The arrow indicates initiation of perfusion of the cis compartment to remove excess PA that had not inserted into the membrane. (D) Monitoring of pore insertion by macroscopic current over time of [WT]6[D425A]1 upon addition to planar lipid bilayers (voltage +20 mV and pH 5.5). The values shown at the arrows represent incremental amounts of [WT]6[D425A]1 added at those time points, giving a total of >200 pmol.
