Figure 4.
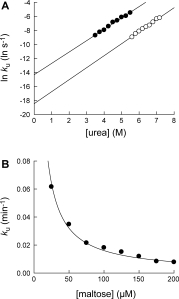
Determination of unfolding kinetic constants of MBP•maltose complex. (A) The effect of urea on unfolding kinetic constants of MBP•maltose complex (○). The kinetic m-value and kunf(H2O) of MBP•maltose complex were determined to be 1.01 ± 0.03 kcal/(mol M) and 9.4 × 10−9 s−1, respectively. Unfolding kinetic constants of MBP in the absence of maltose (•) is shown for comparison. (B) The effect of maltose concentration on unfolding kinetic constants of MBP•maltose complex in 6.0M urea. The maltose concentration is the total maltose concentration in the reaction. By fitting the curve to Eq. (2), the dissociation equilibrium constant (Kd) of the complex was determined to be 2.7 ± 0.3 μM.
