Figure 1.
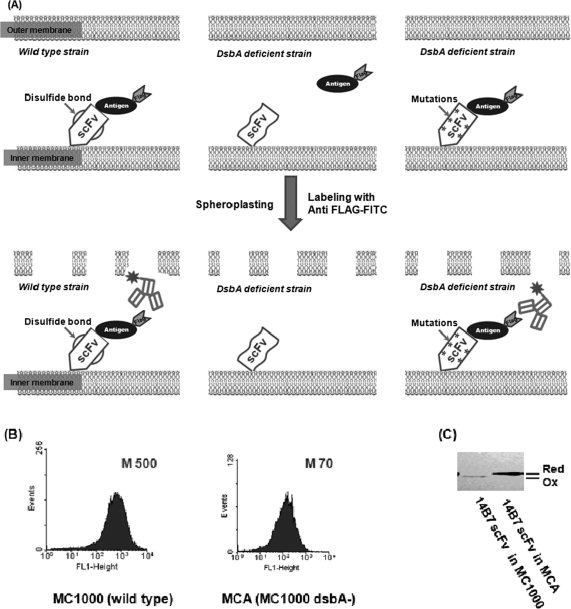
Isolation of active antibody fragments in E. coli dsbA mutants. (A) Schematic showing the screening strategy. Left panel: a correctly folded scFv anchored on the inner membrane of wild-type cells is able to bind antigen. A fluorescent antibody that recognizes an epitope tag on the antigen is used to detect the formation of antibody:antigen complex. Middle panel: in the dsbA mutant, the scFv is reduced and cannot bind antigen. Upon spheroplasting, the antigen diffuses away and hence the cell is not labeled by the fluorescent anti-epitope tag antibody. Right panel: a mutant scFv that is capable of folding in the absence of disulfide bonds can bind antigen in dsbA cells. M, mean fluorescence intensity of the cell population. (B) Fluorescence histograms of cells coexpressing the 14B7* scFv and PA domain IV proteins in either E. coli MC1000 (wild-type) or MCA (MC1000 dsbA). The formation of the antibody:antigen complex was detected by labeling with 200 nM anti-FLAG-FITC. M, mean fluorescent intensity. (C) The redox state of the 14B7* scFv in MC1000 or MCA cells following alkylation with AMS and separation by nonreducing SDS-PAGE.
