Figure 4.
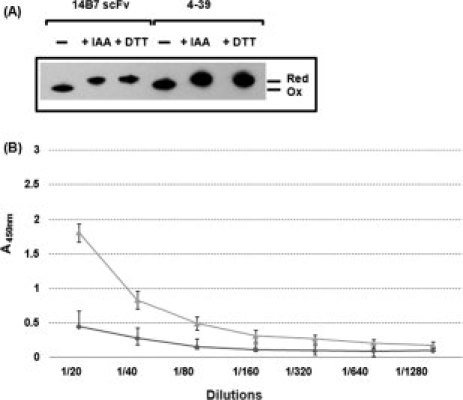
Cysteine redox state and antigen binding activity of scFvs expressed in the E. coli cytoplasm. (A) Cysteine redox state in cytoplasmically expressed scFvs. Upon harvesting, cells were incubated either with 100 mM of prechilled IAA or with the same concentration of DTT for 20 min on ice. The cells were pelleted, lysed in a French press, the cell debris was removed, and the soluble proteins were precipitated by 10% TCA. The precipitated proteins were resuspended in buffer containing AMS. The electrophoretic mobility of scFv proteins was analyzed by nonreducing SDS-PAGE and Western blotting using anti-His-HRP at a 1:10,000 dilution. (B) ELISA: Before lysis, cells were treated with 100 mM DTT, washed with PBS, lysed in a French press, and the soluble proteins were applied to a PA-coated ELISA plate. The bound scFvs were detected by 50 μL of 1:10,000 diluted anti-His-HRP conjugate. ( : 14B7* scFv with 100 mM DTT,
: 14B7* scFv with 100 mM DTT,  : 4-39 scFv with 100 mM DTT).
: 4-39 scFv with 100 mM DTT).
