Abstract
The combination of intra-arterial low-dose cisplatin and 5-fluorouracil (5-FU) is effective against advanced hepatocellular carcinoma (HCC). Systemic gemcitabine chemotherapy seems effective in many cancers. We report the results of combination therapy with systemic gemcitabine, intra-arterial low-dose cisplatin and 5-FU (GEMFP). Seven patients with non-resectable advanced HCC were treated with GEMFP. One course of chemotherapy consisted of daily intra-arterial cisplatin (20 mg/body weight/hour on d 1, 10 mg/body weight per 0.5 h on d 2-5 and 8-12), followed by 5-FU (250 mg/body weight per 5 h on d 1-5 and 8-12) via an injection port. Gemcitabine at 1000 mg/m2 was administered intravenously at 0.5 h on d 1 and 8. The objective response was 57%. The response to GEMFP was as follows: complete response (no patients), partial response (four patients), stable disease (three patients), and progressive disease (no patients). The median survival period was 8 mo (range, 5-55). With regard to the National Cancer Institute Common Toxicity Criteria (NCI-CTC) grade 3 or 4 adverse reactions, seven (100%), seven, six (86%) and one (14%) patients developed leukopenia, neutropenia, thrombocytopenia and anemia, respectively. GEMFP may potentially be effective for non-resectable advanced HCC, but it has severe hematologic toxicity.
Keywords: 5-fluorouracil, Cisplatin, Gemcitabine, Hepatocellular carcinoma
INTRODUCTION
Hepatocellular carcinoma (HCC) is one of the most common neoplasms in Africa and Asia, including Japan[1–3]. Despite advances in diagnostic techniques and therapeutic procedures [e.g. ultrasonography (US), computed tomography (CT), magnetic resonance imaging (MRI), angiography (AG), surgical resection, radiofrequency ablation (RFA), percutaneous ethanol injection (PEI), and transcatheter arterial chemoembolization (TACE)], morbidity [e.g. portal vein tumor thrombosis (PVTT) and distant metastasis], mortality rates for non-resectable advanced HCC remain poor[4–8].
Advances in implantable drug delivery systems have made it possible to administer repeated arterial infusion of anticancer agents. Initial attempts have included monotherapy with intra-arterial 5-fluorouracil (5-FU) for non-resectable HCC[9,10]. However, such treatment results in a low response rate (13% and 22%). Several groups have used low-dose cisplatin and 5-FU for advanced HCC with PVTT, and have reported favorable results[11–13]. Several recent studies have reported the survival benefits of combination therapy of intra-arterial 5-FU and subcutaneous interferon alpha (IFN-α) therapy for advanced HCC with PVTT[14–18].
Gemcitabine is a novel nucleoside analog with a broad spectrum of antitumor activity in preclinical murine leukemia and solid tumor models[19–23]. Several studies have reported the efficiency of intravenous gemcitabine alone and in combination with other anticancer agents for advanced HCC[21–23]. Such regimens have resulted in response rates ranging from 17.8% to 20.0%. When considered with the results of the above chemotherapy, systemic gemcitabine combined with intra-arterial chemotherapy may potentially be useful for patients with advanced HCC. However, to the best of our knowledge, there is no information about systemic gemcitabine combined with intra-arterial low-dose cisplatin and 5-FU (GEMFP) for advanced HCC. We report here the efficacy and safety of GEMFP in the treatment of seven patients with advanced HCC.
CASE REPORT
Eligibility
The treatment eligibility criteria were as follows: (1) Age 20-70 years; (2) Child-Pugh class A; (3) leukocyte count > 3500/μL; (4) neutrophil count > 2000/μL; (5) hemoglobin > 12 g/dL; (6) platelet count > 65 000/μL; (7) total bilirubin < 2.0 mg/dL; (8) serum creatinine < 1.0 mg/dL; (9) aminotransferase < 100 IU/L (10) non-resectable HCC or not suitable for local ablation therapy because of multiple tumors or PVTT (in the first branch or trunk); (11) HCC not suitable for TACE or TACE was ineffective; (12) main tumor size > 40 mm; (13) tumor number > 5; (14) bilobular lesions; (15) Eastern Cooperative Oncology Group performance status (PS) of 0[24]; (16) HCC without marked arteriovenous shunt; (17) HCC without marked arterioportal shunt; (18) no extra-hepatic metastases; (19) absence of other malignant diseases; (20) no recent history of upper gastrointestinal bleeding; (21) no history of heavy alcohol abuse; and (22) no other serious medical condition that would interfere with participation in this study. Participation also required signing informed consent to the study, which had been pre-approved by the Institutional Review Board of Hiroshima University.
Patients
From June 2002 to December 2006, 282 consecutive patients with non-resectable HCC were admitted to our hospital. Due to the progression of HCC (e.g. PVTT, extrahepatic metastases), these patients were not suitable candidates for either surgical resection or local ablation therapy, including RFA and PEI. Diagnosis of HCC was established based on typical hypervascular radiological features or histopathological examination of needle biopsy specimens. HCC was also assessed by US, CT and AG. Furthermore, CT was obtained during arterial portography and computerized tomographic hepatic arteriography. Further assessment of HCC was conducted by measuring α-fetoprotein (AFP) and Des-γ-carboxy prothrombin (DCP). PVTT grading based on the location of the tumor thrombus was determined according to the criteria of the Liver Cancer Study Group of Japan[25]. Of the 282 patients with advanced HCC, seven agreed to be treated with GEMFP. Table 1 lists the baseline profiles, response, and treatment outcomes. The seven patients were assessed retrospectively.
Table 1.
Baseline profiles, response, and outcome of seven patients with HCC
| Case | Age(yr) | Sex | PS | Hepatitis | Child-Pugh class | AFP (ng/mL) | L3 (%) | DCP (mAU/mL) | Main tumor size (mm) | Main tumor morphology | Vascular invasion | Tumor location | Tumor volume | Previous treatment | Treatment cycles | Response | Outcome (mo) | Cause of death |
| 1 | 40 | M | 0 | HBV | A | 934.3 | 15.9 | 333 | 50 | Massive | Vp3/Vv0 | Bilobular, multiple | < 50% | None | 2 | PR | 55, alive | |
| 2 | 35 | M | 0 | HBV | A | 593 350 | 1.9 | > 2000 | 130 | Massive | Vp4/Vv3 | Bilobular, multiple | ≥ 50% | HAI | 3 | PR | 10, dead | Respiratory failure due to lung metastases |
| 3 | 51 | M | 0 | HBV | A | 33 460 | 78 | > 2000 | 80 | Massive | Vp3/Vv0 | Bilobular, multiple | < 50% | HAI | 3 | PR | 8, dead | SAH due to a cerebral aneurysm |
| 4 | 60 | M | 0 | HCV | A | 12.4 | 35.5 | > 2000 | 45 | Massive | Vp3/Vv0 | Bilobular, multiple | ≥ 50% | HAI | 1 | PR | 6, dead | Intrahepatic HCC related liver failure |
| 5 | 68 | M | 0 | HCV | A | 7.5 | < 0.5 | 86 | 42 | Nodular | Vp0/Vv0 | Bilobular, multiple | < 50% | TACE | 1 | SD | 25, dead | Aspiration-related pneumonia due to vertebra metastasis |
| 6 | 64 | M | 0 | HCV | A | 127 920 | 64.3 | 698 | 45 | Nodular | Vp3/Vv0 | Bilobular, multiple | < 50% | TACE | 2 | SD | 7, dead | Intrahepatic HCC related liver failure |
| 7 | 42 | M | 0 | HBV | A | 372.8 | 67.5 | 15 | 100 | Diffuse | Vp4/Vv0 | Bilobular, multiple | ≥ 50% | TACE | 2 | SD | 5, dead | Intrahepatic HCC related liver failure |
Vp0: No PVTT; Vp3: PVTT in the first branch; Vp4: PVTT in the trunk; Vv0: No hepatic venous tumor thrombus; Vv3: Hepatic venous tumor thrombus in inferior vena cava; Multiple lesions; massive type HCC; Nodular, nodular type HCC; diffuse type HCC; Bilobular: Lesions; HAI: Hepatic arterial infusion chemotherapy of low-dose cisplatin and 5-FU.
Treatment protocol
One course of chemotherapy lasted for 2 wk. Patients received repeated arterial infusions of anticancer agents (5-FU and cisplatin) via an injection port. One course of chemotherapy consisted of daily intra-arterial cisplatin (20 mg/body weight on d 1, 10 mg/body weight on d 2-5 and 8-12), followed by 5-FU (250 mg/body weight on d 1-5 and 8-12). D 6 and 7 were a rest period. Cisplatin and 5-FU were administered by a mechanical infusion pump at 20 mg/h and 250 mg/5 h, respectively. Gemcitabine at 1000 mg/m2 was administered intravenously at 0.5 h on d 1 and 8. Ondansetron hydrochloride, a serotonin antagonist, was administered intravenously. Intravenous hydration was provided by saline infusion (1000 mL) to prevent nephrotoxicity during chemotherapy. In principle, GEMFP was repeated for several courses until evidence of progressive disease, worsening of PS, worsening of hepatic reserve function, unacceptable toxicity, or patient refusal to continue. A 4-8-wk rest period of no treatment was allowed after each treatment course.
Evaluation
The maximum response to treatment was assessed in all seven patients. The response was defined according to the criteria of the Response Evaluation Criteria in Solid Tumors (RECIST)[26]. Adverse reactions were assessed every week during and after treatment using the National Cancer Institute Common Toxicity Criteria (NCI-CTC) (version 2.0)[27].
Response and outcomes
The objective response was 57% (Table 1). No patients showed a complete response (CR) to GEMFP and four patients showed a partial response (PR). Three patients achieved stable disease (SD) (Cases 5-7). No patient showed progressive disease (PD). In the four patients with a PR (Cases 1-4), marked regression of tumor and a decrease in tumor markers were observed after the initiation of GEMFP. The median survival period was 8 mo (range, 5-55 mo). One patient was still alive (55 mo) at the end of the observation period (Case 1). Six patients died during the observation period. Three patients died of intrahepatic HCC-related liver failure (Cases 4, 6 and 7), one from respiratory failure associated with lung metastases (Case 2), one from subarachnoid hemorrhage (SAH) (Case 3), and one from aspiration-related pneumonia due to vertebra metastasis (Case 5). Each clinical course is described in detail below.
Case 1: This patient is still alive without recurrence of HCC at the end of the observation period. The patient received two courses of GEMFP and achieved PR at 1.5 mo after the initiation of GEMFP. He then underwent surgical resection for remnant HCC, and histopathological examination of the excised tumor showed HCC with massive coagulative necrosis. Six months after surgical resection, a recurrent tumor appeared in subsegment 6, with a diameter of 15 mm. The tumor was treated with RFA. No other recurrence has been detected so far.
Case 2: A-35-year-old man with Hepatitis B virus (HBV)-related chronic hepatitis and massive HCC (130 mm primary tumor in right hepatic lobe) and multiple intrahepatic metastases associated with PVTT in the trunk, and hepatic venous tumor thrombus in the inferior vena cava was admitted to our hospital. He was considered not suitable for surgical resection, local ablation therapy, or TACE because of the far advanced HCC. He was first treated with one course of intra-arterial low-dose cisplatin and 5-FU, which resulted in PD. Next, he received three courses of GEMFP. At 3 mo after the initiation of GEMFP, the levels of AFP and lectin-reactive AFP (AFP-L3) decreased from 593 350 to 66 450 ng/mL and 21 to < 0.5%, respectively. Repeat CT scans showed regression of the primary tumors, PVTT and hepatic venous tumor thrombus (Figures 1, 2). Nevertheless, the patient developed severe leukopenia, neutropenia, thrombocytopenia, and anemia, which required treatment with granulocyte colony-stimulating factor (G-CSF), and platelet and blood transfusion. No bleeding tendency or infectious disease was observed. One month after the end of the three courses of GEMFP (4 mo after the initiation of GEMFP), a CT scan showed multiple lung metastases. PR was persistently seen with respect to the intrahepatic HCC. Eight months after the initiation of GEMFP, brain metastases were identified. Subsequently, the patient received systemic chemotherapy but it was ineffective. Finally, 10 mo after the initiation of GEMFP, he died of respiratory failure due to lung metastases. Regrowth of intrahepatic HCC was not observed during the follow-up period.
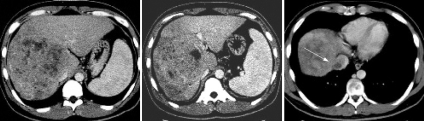
Figure 1.
A CT scan showing massive HCC with PVTT in the trunk and hepatic venous tumor thrombus in the inferior vena cava (arrow). Primary tumor diameter was 130 mm in the right hepatic lobe. No portal blood flow was observed in the right first branch to the trunk.
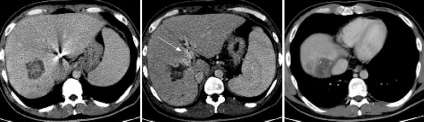
Figure 2.
After three courses of GEMFP, CT scanning showed marked regression of the primary tumor (55 mm) with demonstrable portal blood flow (arrow) and the disappearance of hepatic venous tumor thrombus in the inferior vena cava.
Case 3: This patient was treated with three courses of GEMFP. After one course, 1 mo after the initiation of GEMFP, he achieved PR. He continued to be treated with GEMFP and remained in PR. One month after completion of three courses of GEMFP, 8 mo after the initiation of GEMFP, he died of SAH due to rupture of a cerebral aneurysm. Because bleeding tendency and hematologic toxicity were not observed at the onset of SAH, the relationship between cause of death and GEMFP could not be confirmed.
Case 4: This patient received one course of GEMFP. One month after the initiation of GEMFP, he achieved PR. He required a long (2 mo) rest period from GEMFP because of severe leukopenia, neutropenia and thrombocytopenia. During that period, regrowth of HCC appeared on CT scan. Hepatic reserve function rapidly deteriorated because of the extent of the tumor. No further chemotherapy could be used because of poor hepatic reserve function. Six months after the initiation of GEMFP, he died of intrahepatic HCC-related liver failure.
Case 5: This patient was treated with one course of GEMFP. He subsequently achieved SD, 1 mo after the initiation of GEMFP. The treatment protocol was modified (intra-arterial low-dose cisplatin and 5-FU) after the single course of GEMFP because of severe thrombocytopenia. He continued to be treated for HCC, and 14 mo after initiation of GEMFP, vertebral metastasis was noticed. The patient developed complete spinal cord injury due to vertebral metastasis, with gradual worsening of PS. Finally, PS changed to 4 and he died of aspiration-related pneumonia. He survived for 25 mo after the initiation of GEMFP.
Case 6: This patient received two courses of GEMFP and achieved SD after one course, 1 mo after the initiation of GEMFP. He continued to receive GEMFP. After 2 courses of GEMFP, 5 mo after initiation of GEMFP, CT scan showed progression of HCC and laboratory tests showed associated worsening of hepatic reserve function. Six month after the initiation of GEMFP, spontaneous rupture of HCC occurred suddenly. This resulted in progressive hepatic reserve dysfunction. Finally, he died of intrahepatic HCC-related liver failure 7 mo after the initiation of GEMFP.
Case 7: This patient received two courses of GEMFP, and achieved SD after 1 course, 1 mo after the initiation of GEMFP. He continued to be treated with GEMFP. After two courses of GEMFP, 4 mo after initiation of GEMFP, progression of HCC was noted on CT scan, together with associated obstructive jaundice and progressive hepatic reserve dysfunction. Finally, he died of intrahepatic HCC related liver failure 5 mo after initiation of GEMFP.
Adverse reactions
Table 2 summarizes the adverse reactions encountered during and after GEMFP treatment. No complications arising from catheter implantation and injection port were noted. Nausea, anorexia and anemia were mostly NCI-CTC grade 1 or 2 adverse reactions. With regard to NCI-CTC grade 3 or 4 adverse reactions, seven, seven, six and one patients developed leukopenia, neutropenia, thrombocytopenia and anemia, respectively. Four patients (Cases 2, 4, 6 and 7) required administration of G-CSF. Five patients (Cases 1-4 and 7) required platelet transfusion, and one (Case 2) required both blood and platelet transfusion. No patients developed bleeding tendency, gastrointestinal bleeding, deterioration of hepatic function, renal damage or infectious disease.
Table 2.
NCI-CTC grade of adverse reactions during and after GEMFP
| Case | Leukocyte count (pre/end, /μL) | Neutrophil count (pre/end, /μL) | Hemoglobin (pre/end, g/dL) | Platelet count (pre/end, × 104) | Nausea | Anorexia |
| 1 | 3 (5050/1670) | 3 (3232/835) | 0 (14.6/11.8) | 4 (7.5/1.5) | 0 | 1 |
| 2 | 4 (7260/640) | 4 (5520/420) | 4 (15.7/6.2) | 4 (14.8/1.8) | 0 | 0 |
| 3 | 3 (3950/1720) | 3 (2489/774) | 0 (14.0/12.0) | 4 (6.9/1.7) | 0 | 0 |
| 4 | 3 (7950/1430) | 4 (5168/352) | 0 (16.1/11.7) | 4 (20.7/2.0) | 0 | 0 |
| 5 | 3 (3990/1700) | 3 (2993/840) | 0 (14.6/11.3) | 3 (9.6/4.0) | 1 | 0 |
| 6 | 3 (3840/1040) | 4 (2380/357) | 2 (15.2/8.5) | 4 (15.3/0.5) | 0 | 1 |
| 7 | 3 (6790/1950) | 4 (3870/468) | 2 (12.4/9.6) | 2 (13.9/6.4) | 0 | 0 |
DISCUSSION
The prognosis of patients with advanced HCC complicated with PVTT remains poor, particularly in those with PVTT in the first branches or the trunk. The median survival time of HCC patients with PVTT in the trunk is reported to be about 90 d with supportive care[28]. Three recent studies have reported the efficacy and survival benefits of combination therapy of intra-arterial low-dose cisplatin and 5-FU for patients with advanced HCC[11–13]. These studies included nine, 48 and 18 patients with advanced HCC and PVTT (in the second branch, first branch, or trunk), who showed objective response rates of 44% (4/9 patients), 48% (23/48 patients) and 33% (6/18 patients), respectively. The cumulative survival rates were 40% at 36 mo, 25% at 36 mo, and 28% at 12 mo, respectively. Furthermore, there has been a study of combination therapy with intra-arterial high-dose 5-FU and cisplatin in 41 patients with advanced HCC[29]. Objective response rate was 22% (9/41) and the cumulative survival rate was 47% at 12 mo.
A phase II study of intravenous gemcitabine monotherapy in 28 patients with non-resectable advanced and large HCC (> 10 cm) in 17 patients, extrahepatic metastases in nine, and PVTT in the trunk in 11, and the objective response rate was 18% (5/28 patients)[21]. The median survival time of all patients treated with intravenous gemcitabine monotherapy was 19 wk, and 35 wk for those who achieved an objective response. The objective response rate was 18% (six patients) in another phase II study of intravenous gemcitabine plus intravenous oxaliplatin in 34 patients with non-resectable advanced HCC (10 patients with lung metastases, 12 with PVTT, and seven with a PS of 2)[22], and their median survival time was 12 mo (range, 9-14 mo).
In the present study of GEMFP, the objective response rate was 57%. Compared with the objective response rates of the above studies of intra-arterial low-dose cisplatin and 5-FU (33%-48%), intravenous gemcitabine treatment (18%), and intravenous gemcitabine plus intravenous oxaliplatin treatment (18%), the objective response rate of GEMFP for advanced HCC seems better and more satisfactory. The objective response rate for intra-arterial 5-FU and subcutaneous IFN-α therapy for advanced HCC was reported to be 29% in our hospital[18]. The objective response rate for GEMFP might be favorable compared to that for intra-arterial 5-FU and subcutaneous IFN-α therapy. The current study had a small sample size. So, a further larger, prospective randomized trial is worth considering for assessing combination therapy in patients with advanced HCC. Each chemotherapeutic agent (gemcitabine, 5-FU and cisplatin) has an antitumor effect. Cisplatin has a synergistic effect as a modulator of 5-FU[30–32]. Although the mechanism is not clear, addition of gemcitabine might have a more potent antineoplastic effect compared with intra-arterial low-dose cisplatin and 5-FU alone. Gemcitabine may also have a biomodulator effect that enhances the antineoplastic activity of 5-FU. Gemcitabine and 5-FU might synergize each other’s antineoplastic effects. Cases 2-4 had been treated with low-dose cisplatin and 5-FU before the present study, but all three showed PD. Then, the treatment protocol was changed to GEMFP, and all achieved a PR. Thus, addition of gemcitabine seemed to produce beneficial effects in these patients.
With regard to the adverse reactions to intra-arterial low-dose cisplatin and 5-FU, nausea, loss of appetite, peptic ulcer, leukopenia, thrombocytopenia, deterioration of hepatic function, and renal damage have been reported in previous studies[11–13]. Most of these adverse reactions were considered to be relatively mild and no patient required administration of G-CSF or blood transfusion. With regard to the adverse reactions associated with intravenous gemcitabine monotherapy, leukopenia, anemia, thrombocytopenia, nausea, vomiting, stomatitis, diarrhea, alopecia, skin rash, and fatigue have been reported[21], although they were mostly mild in nature. With regard to NCI-CTC grade 3 or 4 adverse reactions associated with intravenous gemcitabine monotherapy, leukopenia (11%), anemia (14%), thrombocytopenia (11%) and deterioration of hepatic function (14%) have been reported. As for adverse reactions with intravenous gemcitabine plus intravenous oxaliplatin combination therapy, neutropenia, anemia, thrombocytopenia, neurotoxicity, nausea, vomiting, stomatitis, diarrhea, alopecia, and hand-foot syndrome have been reported[22], which were mostly mild. With regard to NCI-CTC grade 3 or 4 adverse reactions associated with intravenous gemcitabine plus intravenous oxaliplatin combination therapy, neutropenia (24%), anemia (9%), thrombocytopenia (27%), and neurotoxicity (9%) have been reported. In the present study, hematologic toxicity was the most severe. With regard to NCI-CTC grade 3 or 4 adverse reactions, seven, seven, six and one patients developed leukopenia, neutropenia, thrombocytopenia and anemia, respectively. Compared with adverse reactions reported with intra-arterial low-dose cisplatin and 5-FU and systemic gemcitabine, the severe hematologic toxicity noted with GEMFP was considered to be mainly due to gemcitabine. However, hematologic toxicity was more severe than that seen with the above systemic chemotherapy using gemcitabine. The combination of intra-arterial low-dose cisplatin, 5-FU and gemcitabine might cause severe hematologic toxicity. Close monitoring of hematologic toxicity is very important with GEMFP. In our patients, Child-Pugh class was A and PS was 0. These limitations in part account for the good tolerance results. HCC patients with Child-Pugh class B or C, and PS > 1 might discontinue this treatment protocol.
The median survival period was 8 mo (range, 5-55 mo) in this study. The median survival of intra-arterial 5-FU and subcutaneous IFN-α therapy for advanced HCC has been reported to be 9 mo in our hospital[18]. Four of the seven patients achieved PR. With regard to three of the four patients with PR, intrahepatic HCC was well controlled during the observation period. However, extrahepatic metastases occurred in one of these patients, and he died of respiratory failure due to lung metastases (Case 2). Although intrahepatic HCC was well controlled by GEMFP, GEMFP was ineffective for extrahepatic metastases. GEMFP in this study seemed to show a poor effect on extrahepatic metastases. However, this study had a small sample size. So, further, larger studies are needed to assess GEMFP. Effective treatment against extrahepatic metastases is needed. Intrahepatic HCC was not well controlled in another patient with PR (Case 4). He died of intrahepatic HCC-related liver failure, 6 mo after the initiation of GEMFP. This survival period was unsatisfactory. This was probably mainly due to the long rest period from GEMFP therapy because of severe hematologic toxicity, during which regrowth of HCC occurred. Tolerability is as important as survival. Modification of the treatment protocol might be considered to avoid hematologic toxicity.
In conclusion, we reported seven cases in which GEMFP may have been an important component of the basic therapeutic regimen for non-resectable advanced HCC with Child-Pugh class A. Although bleeding tendency or infectious disease did not happen, hematologic toxicity was severe in our study. G-CSF and/or platelet transfusion were frequently required. A modified treatment protocol (e.g. dose reduction of gemcitabine) or inclusion criteria (e.g. leukocyte count > 5000/μL, neutrophil count > 3000/μL, hemoglobin > 12 g/dL and platelet count > 100 000/μL) or supportive treatment protocol using G-CSF and/or blood transfusion should be examined to avoid hematologic toxicity. Further studies are needed, including long-term follow-up, cost-benefit, and larger sample size to assess GEMFP-based chemotherapy.
Peer reviewers: Luis Rodrigo, Professor, Gastroenterology Service, Hospital Central de Asturias, c/Celestino Villamil, s.n., Oviedo 33.006, Spain; Mitsuo Shimada, Professor, Department of Digestive and Pediatric Surgery, Tokushima University, Kuramoto 3-18-15, Tokushima 770-8503, Japan; Kazuhiro Hanazaki, MD, Professor and Chairman, Department of Surgery, Kochi Medical School, Kochi University, Kohasu, Okohcho, Nankoku, Kochi 783-8505, Japan
S- Editor Zhong XY L- Editor Kerr C E- Editor Liu Y
References
- 1.Kobayashi M, Ikeda K, Hosaka T, Sezaki H, Someya T, Akuta N, Suzuki F, Suzuki Y, Saitoh S, Arase Y, et al. Natural history of compensated cirrhosis in the Child-Pugh class A compared between 490 patients with hepatitis C and 167 with B virus infections. J Med Virol. 2006;78:459–465. doi: 10.1002/jmv.20562. [DOI] [PubMed] [Google Scholar]
- 2.Okuda K, Fujimoto I, Hanai A, Urano Y. Changing incidence of hepatocellular carcinoma in Japan. Cancer Res. 1987;47:4967–4972. [PubMed] [Google Scholar]
- 3.Health and Welfare Statistics Association. Journal of Health and Welfare Statistics. Tokyo: Health and Welfare Statistics Association. 2000;47:421. [Google Scholar]
- 4.A new prognostic system for hepatocellular carcinoma: a retrospective study of 435 patients: the Cancer of the Liver Italian Program (CLIP) investigators. Hepatology. 1998;28:751–755. doi: 10.1002/hep.510280322. [DOI] [PubMed] [Google Scholar]
- 5.Llovet JM, Bustamante J, Castells A, Vilana R, Ayuso Mdel C, Sala M, Bru C, Rodes J, Bruix J. Natural history of untreated nonsurgical hepatocellular carcinoma: rationale for the design and evaluation of therapeutic trials. Hepatology. 1999;29:62–67. doi: 10.1002/hep.510290145. [DOI] [PubMed] [Google Scholar]
- 6.Uka K, Aikata H, Takaki S, Shirakawa H, Jeong SC, Yamashina K, Hiramatsu A, Kodama H, Takahashi S, Chayama K. Clinical features and prognosis of patients with extrahepatic metastases from hepatocellular carcinoma. World J Gastroenterol. 2007;13:414–420. doi: 10.3748/wjg.v13.i3.414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kamada K, Kitamoto M, Aikata H, Kawakami Y, Kono H, Imamura M, Nakanishi T, Chayama K. Combination of transcatheter arterial chemoembolization using cisplatin-lipiodol suspension and percutaneous ethanol injection for treatment of advanced small hepatocellular carcinoma. Am J Surg. 2002;184:284–290. doi: 10.1016/s0002-9610(02)00933-9. [DOI] [PubMed] [Google Scholar]
- 8.Friedman MA. Primary hepatocellular cancer--present results and future prospects. Int J Radiat Oncol Biol Phys. 1983;9:1841–1850. doi: 10.1016/0360-3016(83)90352-8. [DOI] [PubMed] [Google Scholar]
- 9.Stehlin JS Jr, de Ipolyi PD, Greeff PJ, McGaff CJ Jr, Davis BR, McNary L. Treatment of cancer of the liver. Twenty years' experience with infusion and resection in 414 patients. Ann Surg. 1988;208:23–35. doi: 10.1097/00000658-198807000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Doci R, Bignami P, Bozzetti F, Bonfanti G, Audisio R, Colombo M, Gennari L. Intrahepatic chemotherapy for unresectable hepatocellular carcinoma. Cancer. 1988;61:1983–1987. doi: 10.1002/1097-0142(19880515)61:10<1983::aid-cncr2820611009>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 11.Ando E, Yamashita F, Tanaka M, Tanikawa K. A novel chemotherapy for advanced hepatocellular carcinoma with tumor thrombosis of the main trunk of the portal vein. Cancer. 1997;79:1890–1896. doi: 10.1002/(sici)1097-0142(19970515)79:10<1890::aid-cncr8>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 12.Ando E, Tanaka M, Yamashita F, Kuromatsu R, Yutani S, Fukumori K, Sumie S, Yano Y, Okuda K, Sata M. Hepatic arterial infusion chemotherapy for advanced hepatocellular carcinoma with portal vein tumor thrombosis: analysis of 48 cases. Cancer. 2002;95:588–595. doi: 10.1002/cncr.10694. [DOI] [PubMed] [Google Scholar]
- 13.Lai YC, Shih CY, Jeng CM, Yang SS, Hu JT, Sung YC, Liu HT, Hou SM, Wu CH, Chen TK. Hepatic arterial infusion chemotherapy for hepatocellular carcinoma with portal vein tumor thrombosis. World J Gastroenterol. 2003;9:2666–2670. doi: 10.3748/wjg.v9.i12.2666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sakon M, Nagano H, Dono K, Nakamori S, Umeshita K, Yamada A, Kawata S, Imai Y, Iijima S, Monden M. Combined intraarterial 5-fluorouracil and subcutaneous interferon-alpha therapy for advanced hepatocellular carcinoma with tumor thrombi in the major portal branches. Cancer. 2002;94:435–442. doi: 10.1002/cncr.10246. [DOI] [PubMed] [Google Scholar]
- 15.Ota H, Nagano H, Sakon M, Eguchi H, Kondo M, Yamamoto T, Nakamura M, Damdinsuren B, Wada H, Marubashi S, et al. Treatment of hepatocellular carcinoma with major portal vein thrombosis by combined therapy with subcutaneous interferon-alpha and intra-arterial 5-fluorouracil; role of type 1 interferon receptor expression. Br J Cancer. 2005;93:557–564. doi: 10.1038/sj.bjc.6602742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Obi S, Yoshida H, Toune R, Unuma T, Kanda M, Sato S, Tateishi R, Teratani T, Shiina S, Omata M. Combination therapy of intraarterial 5-fluorouracil and systemic interferon-alpha for advanced hepatocellular carcinoma with portal venous invasion. Cancer. 2006;106:1990–1997. doi: 10.1002/cncr.21832. [DOI] [PubMed] [Google Scholar]
- 17.Uka K, Aikata H, Takaki S, Miki D, Jeong SC, Hiramatsu A, Kodama H, Shirakawa H, Kawakami Y, Takahashi S, et al. Similar effects of recombinant interferon-alpha-2b and natural interferon-alpha when combined with intra-arterial 5-fluorouracil for the treatment of advanced hepatocellular carcinoma. Liver Int. 2007;27:1209–1216. doi: 10.1111/j.1478-3231.2007.01554.x. [DOI] [PubMed] [Google Scholar]
- 18.Uka K, Aikata H, Takaki S, Miki D, Kawaoka T, Jeong SC, Takahashi S, Toyota N, Ito K, Chayama K. Pretreatment predictor of response, time to progression, and survival to intraarterial 5-fluorouracil/interferon combination therapy in patients with advanced hepatocellular carcinoma. J Gastroenterol. 2007;42:845–853. doi: 10.1007/s00535-007-2099-8. [DOI] [PubMed] [Google Scholar]
- 19.Gridney GB, Boder GB, Hertel LW. Antitumor activity of 2’, 2’-difluorodeoxycytidine (LY 188011) Proc Am Assoc Cancer Res. 1986;27:296–301. [Google Scholar]
- 20.Boven E, Schipper H, Erkelens CA, Hatty SA, Pinedo HM. The influence of the schedule and the dose of gemcitabine on the anti-tumour efficacy in experimental human cancer. Br J Cancer. 1993;68:52–56. doi: 10.1038/bjc.1993.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang TS, Lin YC, Chen JS, Wang HM, Wang CH. Phase II study of gemcitabine in patients with advanced hepatocellular carcinoma. Cancer. 2000;89:750–756. doi: 10.1002/1097-0142(20000815)89:4<750::aid-cncr5>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 22.Louafi S, Boige V, Ducreux M, Bonyhay L, Mansourbakht T, de Baere T, Asnacios A, Hannoun L, Poynard T, Taieb J. Gemcitabine plus oxaliplatin (GEMOX) in patients with advanced hepatocellular carcinoma (HCC): results of a phase II study. Cancer. 2007;109:1384–1390. doi: 10.1002/cncr.22532. [DOI] [PubMed] [Google Scholar]
- 23.Parikh PM, Fuloria J, Babu G, Doval DC, Awasthy BS, Pai VR, Prabhakaran PS, Benson AB. A phase II study of gemcitabine and cisplatin in patients with advanced hepatocellular carcinoma. Trop Gastroenterol. 2005;26:115–118. [PubMed] [Google Scholar]
- 24.Oken MM, Creech RH, Tormey DC, Horton J, Davis TE, McFadden ET, Carbone PP. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol. 1982;5:649–655. [PubMed] [Google Scholar]
- 25.Liver Cancer Study Group of Japan. The general rules for the clinical and pathological study of primary liver cancer (in Japanese). 4th ed. Tokyo: Kanehara. 2000:19. [Google Scholar]
- 26.Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, van Oosterom AT, Christian MC, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 27.Common Toxicity Criteria and Terminology Criteria for Adverse Events. National Cancer Institution. Available from: URL: http://www.cancer.gov/search/results.aspx. [Google Scholar]
- 28.Lee HS, Kim JS, Choi IJ, Chung JW, Park JH, Kim CY The safety and efficacy of transcatheter arterial chemoembolization in the treatment of patients with hepatocellular carcinoma and main portal vein obstruction. A prospective controlled study. Cancer. 1997;79:2087–2094. [PubMed] [Google Scholar]
- 29.Park JY, Ahn SH, Yoon YJ, Kim JK, Lee HW, Lee do Y, Chon CY, Moon YM, Han KH. Repetitive short-course hepatic arterial infusion chemotherapy with high-dose 5-fluorouracil and cisplatin in patients with advanced hepatocellular carcinoma. Cancer. 2007;110:129–137. doi: 10.1002/cncr.22759. [DOI] [PubMed] [Google Scholar]
- 30.Paquet KJ, Kalk JF, Cuan-Orozco F, Siemens F, Koussouris P, Mercado MA. Hepatic chemoinfusion of 5-FU in metastasis of gastrointestinal cancer and advanced primary hepatocellular carcinoma. Eur J Surg Oncol. 1992;18:156–161. [PubMed] [Google Scholar]
- 31.Scanlon KJ, Newman EM, Lu Y, Priest DG. Biochemical basis for cisplatin and 5-fluorouracil synergism in human ovarian carcinoma cells. Proc Natl Acad Sci USA. 1986;83:8923–8925. doi: 10.1073/pnas.83.23.8923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fernandez Hidalgo O, Gonzalez F, Gil A, Campbell W, Barrajon E, Lacave AJ. 120 hours simultaneous infusion of cisplatin and fluorouracil in metastatic breast cancer. Am J Clin Oncol. 1989;12:397–401. doi: 10.1097/00000421-198910000-00007. [DOI] [PubMed] [Google Scholar]