Abstract
African swine fever (ASF) is an infectious and economically important disease of domestic pigs. There is no vaccine, and so reliable diagnosis is essential for control strategies. The performance of four recombinant ASF virus (ASFV) protein (pK205R, pB602L, p104R, and p54)-based enzyme-linked immunosorbent assays (ELISAs) was evaluated with European porcine field sera that had been established by Office International des Epizooties (OIE)-approved tests to be ASFV negative (n = 119) and ASFV positive (n = 80). The κ values showed that there was almost perfect agreement between the results of the “gold standard” test (immunoblotting) and the results obtained by the p54-specific ELISA (κ = 0.95; 95% confidence interval [CI], 0.90 to 0.99) and the pK205R-specific ELISA or the pB602L-specific ELISA (κ = 0.92; 95% CI, 0.86 to 0.97). For the pA104R-specific ELISA, there was substantial to almost perfect agreement (κ = 0.81; 95% CI, 0.72 to 0.89). Similar results were observed by the OIE-approved ELISA (κ = 0.89; 95% CI, 0.82 to 0.95). Importantly, antibodies against these proteins were detectable early after infection of domestic pigs. Preliminary testing of 9 positive and 17 negative serum samples from pigs from West Africa showed identical results by the recombinant protein-based ELISA and the OIE-approved tests. In contrast, there was a high degree of specificity but a surprisingly a low level of sensitivity with 7 positive and 342 negative serum samples from pigs from East Africa. With poorly preserved sera, only the p104R-specific ELISA showed a significant reduction in sensitivity compared to that of the OIE-approved ELISA. Finally, these recombinant proteins also detected antibodies in the sera of the majority of infected warthogs. Thus, recombinant ASFV proteins p54, pB602L, and pK205R provide sensitive and specific targets for the detection of antibodies in European and West African domestic pigs and warthogs.
African swine fever (ASF) virus (ASFV) is an icosahedral cytoplasmic DNA virus that infects pigs and soft ticks of the Ornithodoros genus. This virus is the sole member of the family Asfarviridae (6). ASFV has variable pathogenicity in domestic pigs, with infections ranging from being highly lethal to subclinical. Infection of wildlife mammalian hosts, the warthog and the bushpig, on the other hand, results in an unapparent, nonpathogenic infection, which provides a potentially dangerous reservoir of virus. There is no vaccine. Therefore, rapid and specific diagnostic procedures are an essential component of any control strategy. In addition, the presence of virus strains with reduced virulence and the resulting presence of asymptomatic infected animals (4, 11) make the serological diagnosis the only realistic basis for the control of the disease in affected countries. As a general rule, pigs that survive natural infection develop antibodies against ASFV from 7 to 10 days after infection. These antibodies persist for long periods of time (16), perhaps due to continuous antigenic stimulation by the frequent occurrence of persistent infection. Thus, antibody detection is a rational approach to the detection of the subacute and chronic forms of the disease.
The role of specific antibodies in immunity to ASFV infection in pigs has been controversial. The passive transfer of anti-ASFV antibodies delays the onset of clinical signs but does not consistently protect animals from eventual death (25, 26, 17). Similarly, vaccination with the putative protective proteins p30 and p54 conferred protection to only 50% of the tested animals (10). In a different study, in which no protection was observed after immunization against p54, p30, and p72, the only effects detected were a delay in the onset of clinical disease and a reduction in the level of viremia (15). Such observations emphasize the role of cell-mediated immune responses during ASFV infection. Indeed, a positive correlation was observed between the stimulation of NK cell activity and the absence of clinical symptoms after experimental infection, suggesting that NK cells play an important role in protective immunity (13). In addition to NK cells, CD8+ T cells may also play a role, as their depletion in vivo abrogates protective immunity to ASFV infection (18). Therefore, immunity to ASFV is likely to be due to a combination of both serological and cellular mechanisms. This complexity of the porcine immune response to ASFV has impaired the development of an effective vaccine but does justify diagnosis on the basis of the detection of antibodies.
Current Office International des Epizooties (OIE)-approved assays for ASFV-specific antibody determination consist of an initial screening of sera by enzyme-linked immunosorbent assay (OIE-ELISA), followed by an immunoblotting assay to confirm the results for samples with doubtful and positive results. These OIE-approved tests are based on the use of live virus as the antigen and involve the requirement of level 3 biosafety facilities for the production and handling of the pathogen (16, 20, 21). The risk associated with the handling of live virus, together with the lack of reliability of the OIE-ELISA for the analysis of poorly preserved samples so often encountered in sera of African origin (1, 3), provides the stimulus for the development of alternative and more robust systems for the detection of anti-ASFV antibodies. Indeed, previous studies have demonstrated that recombinant viral proteins can give improved specificity and sensitivity when they are applied to the analysis of European field sera (9, 19, 22).
In previous studies, 12 serological immunodeterminants of ASFV were characterized by exhaustive screening of a representative lambda phage cDNA expression library of the tissue culture-adapted Ba71V isolate of ASFV for antibodies (12). These included four proteins encoded by previously unassigned open reading frames (ORFs) (B602L, C44L, CP312R, and K205R), as well as some of the more well studied structural proteins (pA104R, p10, p32, p54, and p73) and three enzymes (RNA reductase, DNA ligase, and thymidine kinase). The complete sequence of each of these proteins was then recloned into pGEX for expression in Escherichia coli, followed by purification of the recombinant proteins and testing against sera from experimentally infected animals. Four of these proteins (p54/E183L, histone-like/pA104R, pB602L, and pK205R) were revealed to be promising targets for both immunoglobulin G (IgG) and IgM antibody responses (23), and further validation of these proteins as targets is the focus of this work. The results obtained by the analysis of a large collection of serum samples from susceptible animals from Europe and Africa were comparable to the results obtained by the OIE-ELISA prescribed for use for international trade.
MATERIALS AND METHODS
Cloning, expression, and purification of recombinant proteins.
The cloning, expression, and purification of the recombinant proteins has been described previously (23). Briefly, ORFs A104R, E183L, B602L, and K205R were amplified from the genomic DNA of isolate BA71V (see the next section) with specific primers and were cloned into the pGEX 4T-1 prokaryotic expression vector. The resulting constructs were used to transform competent E. coli cells. The proteins histone-like protein/pA104R, pB602L, and pK205R were recovered from E. coli cultures as soluble proteins on glutathione-Sepharose 4B columns (Amersham-Pharmacia), as described by the manufacturer. The insoluble protein p54/pE183L was purified after solubilization with urea. The glutathione S-transferase (GST) protein was also purified by the same method in order to provide a negative control in the serological assays.
Viruses and virus propagation.
Three ASFV virulent and hemadsorbing isolates were used in this study: Spanish isolates obtained in 1970 (isolate E70) and 1975 (isolate E75) and an African isolate from Uganda obtained in 2003 (isolate Ug03H). Primary leukocyte cultures were used for the propagation and titration of these wild-type viruses, as described previously (14). Briefly, porcine blood monocytes were seeded into 96-well tissue-culture-grade microtiter plates (200 μl; 300,000 cells per well) in homologous swine serum, and the plates were incubated in a humidified atmosphere containing 5% CO2 at 37°C. On day 3, the cultures were infected with ASFV in serial twofold dilutions and the plates were incubated for 24 h at 37°C. After inoculation, a preparation of 1% homologous red blood cells in buffered saline was added to each well. The plates were examined for hemadsorption over a period of 6 days. Titers of ASFV were expressed as the 50% hemadsorbing dose (HAD50) per milliliter.
The E70 virus isolate was adapted to grow in the monkey stable (MS) kidney cell line (ECACC, 91070510) to yield an attenuated virus after 48 passages (E70 MS 48). The attenuated virus was used for antigen production, as described by Escribano et al. (7). The partially attenuated virus, E75 CV14, was obtained by four passages of the E75 virus isolate on CV1 cells (ATCC, CCL 70). Virus E75 CV14 was used for experimental infection and induces protection against the parental virus (24). Finally, isolate BA71V, recovered in Spain in 1971, has been adapted to Vero cells (ATCC CCL 81) after an undetermined number of passages and is no longer pathogenic for pigs. This virus isolate has been sequenced (27) and was used to obtain the viral genomic DNA used as the template for the amplification of ORFs A104R, E183L, B602L, and K205R (see the previous section). Virus infection of MS cells was carried out with Dulbecco's modified Eagle's medium (DMEM) containing 2% swine serum. Virus infection of CV1 and Vero cells was carried out with DMEM containing 2% fetal bovine serum.
Experimental infections.
Experimental infections of Landrace × Large White pigs were carried out at a level 3 biosafety animal facility at CISA-INIA, Madrid, Spain. Two pigs (pigs 2U and 3U) were inoculated by the intramuscular route with 105 HAD50s of the Ug03H ASFV isolate. The severely sick animals were painlessly killed at 9 days postinfection (dpi) (pig 3U) and 12 dpi (pig 2U). Two additional pigs (pigs 1 and 2) were inoculated by the intramuscular route with the attenuated ASFV E75 CV14 (102 50% tissue culture infective doses/ml). One sick animal (pig 1) was painlessly killed at 21 dpi; and the other asymptomatic animal (pig 2) was reinoculated at 30 dpi with homologous virulent isolate E75 (103 HAD50s/ml); then given a third challenge at 45 dpi with heterologous virulent isolate E70 (103 HAD50s/ml), and then painlessly killed at 51 dpi.
Serum samples. (i) Field serum samples.
The pig and warthog sera used in this study consisted of a collection of samples obtained from different locations in Europe and Africa. The European samples were validated by the OIE-approved tests (OIE-ELISA and immunoblotting) and contained 80 ASFV-positive serum samples collected during a series of ASF outbreaks in Spain between 1989 and 1992 and 119 ASFV-negative serum samples collected during the last 10 years in Spain and Germany. A total of 35 of the negative serum samples were previously confirmed to be antibody positive for the classical swine fever virus, and another 25 serum samples were previously confirmed to be antibody positive for swine vesicular disease. An additional 39 field serum samples that initially tested positive by the OIE-approved tests were stored at 37°C for a month to generate poorly preserved sera for repeated analysis by the OIE-approved tests and the recombinant protein-based ELISAs.
The African serum samples were collected from domestic pigs in diverse regions of Africa during different epidemiological and surveillance studies performed in Uganda (n = 254), Mozambique (n = 95), Nigeria (n = 6), and Burkina Faso (n = 20).
Finally, 26 warthog serum samples were collected in Uganda.
(ii) Experimental serum samples.
Sera were collected on days 0, 3, 6, 8, 10, and 12 postinfection from pigs 2U and 3U, which had been inoculated with ASFV isolate Ug03H. Sera were collected on days 0, 10, 15, and 21 postinfection from pig 1, which had been inoculated with attenuated ASFV isolate E75 CV14 (102 50% tissue culture infective doses/ml). Sera were collected on days 0, 10, 15, 21, 30, 36, 45, and 51 postinfection from pig 2, which had first been inoculated with attenuated ASFV isolate E75 CV14 (102 50% tissue culture infective doses/ml), reinoculated at 30 dpi with homologous virulent isolate E75 (103 HAD50s/ml), and then given a third challenge at 45 dpi with heterologous virulent E70 (103 HAD50s/ml).
(iii) Reference serum samples.
Serum from pig 2, taken 7 days after the third challenge (51 dpi), was used as the positive reference serum. The ASFV-negative reference serum sample was obtained from a blood donor pig kept at CISA-INIA.
Conventional ELISA and immunoblotting assays (OIE-approved analyses).
Both conventional ELISA and immunoblotting assays were performed with a lysate of MS cells infected with ASFV E70 MS 48 as the antigen and protein A conjugated to horseradish peroxidase as the indicator. Both procedures were carried out by following the protocols described in the OIE Manual of Diagnostic Tests and Vaccines for Terrestrial Animals (16).
ELISA with recombinant proteins as antigen.
Briefly, microtiter plates (Polysorb immunoplates; Nunc) were incubated at 4°C overnight with 50 μl/well of recombinant proteins (pA104R, p54, pB602L, pK205R) or the GST-negative control at a previously determined optimal concentration in coating buffer (0.1 M carbonate buffer, pH 9.6). The coated plates were washed four times with phosphate-buffered saline (PBS; pH 7.5) containing 0.05% (vol/vol) Tween 20 (PBS-T) and used immediately or stored at −20°C until use. The plates were subsequently blocked with PBS (pH 7.5) containing 5% (wt/vol) skim milk (PBS-M) for 1 h at 37°C. Then, the porcine sera were added at a dilution of 1:200 in PBS-M and incubated for 1 h at 37°C. Positive and negative reference sera were included on each plate. The plates were washed four times with PBS-T, and the horseradish peroxidase-labeled protein A diluted 1:5,000 in PBS-M was added. The plates were incubated for 1 h at 37°C. After the plates were washed, 50 μl of o-phenylenediamine (Sigma) was added to each well. After incubation for 20 min at room temperature, the reaction was stopped by the addition of 50 μl of 3 N H2SO4, and the optical density (OD) was measured at a wavelength of 492 nm. Titers were expressed as the ratio between the ODs obtained for each sample against recombinant antigen-positive and GST negative-control proteins.
Calculation and data analysis.
Sensitivity and specificity were calculated by using the results of immunoblotting as the reference. All serum samples with doubtful results by ELISA (those with results in the cutoff interval) were considered positive. The concordance between each ELISA (the conventional OIE-approved assay and the proposed recombinant protein-based assays) and the OIE confirmatory test (immunoblotting) was calculated as the overall percent agreement between the results of the two assays by using two-by-two contingency tables. κ statistics were used to evaluate the level of agreement between concordant results in excess of that expected by chance, with κ values of 0.81 to 1.00 representing almost perfect agreement, values of 0.61 to 0.80 representing substantial agreement, values of 0.41 to 0.60 representing moderate agreement, values of 0.21 to 0.40 representing fair agreement, values of 0.01 to 0.20 representing slight agreement, and values of 0.00 representing no agreement (8).
RESULTS
Standardization of recombinant protein-based ELISAs.
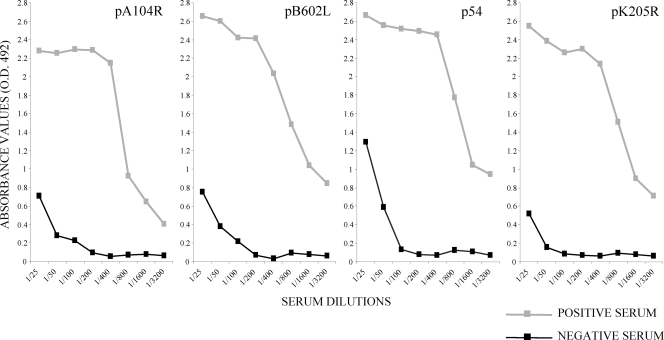
The optimal concentration of recombinant antigens used to coat the ELISA microtiter plates was determined by using the positive and negative reference sera. The absorbance values of the ELISAs with the recombinant antigens were found to be optimal when the microtiter plates were coated with a concentration of 5.0 μg/ml of antigen pB602L, 2.5 μg/ml of antigen p54, 2.0 μg/ml of antigen pA104R, or 1.3 μg/ml of antigen pK205R. No reactivity (OD at 492 nm [OD492], less than 0.2) was detected when the positive reference serum was tested against the GST protein expressed in E. coli or when the negative reference serum was incubated with the recombinant proteins (data not shown). The appropriate dilution of sera for the ELISA was determined by titration to be 1:200. At this dilution, the absorbance corresponding to positive reference sera was 10 times greater than that corresponding to the negative reference serum (Fig. 1).
FIG. 1.
Titration curves of reference sera by ELISAs with recombinant proteins pA104R, pB602L, p54, and pK205R. Sera were tested in serial duplicate twofold dilutions ranging from 1:25 to 1:3,200. The results are expressed as the OD492 and correspond to the average of the values obtained in at least three different analyses.
Comparative study between conventional and recombinant protein-based ELISAs with European field serum samples.
One hundred ninety-nine European porcine serum samples, all from domestic pigs in the field established to be ASFV negative (n = 119) and ASFV positive (n = 80) by OIE-approved tests (ELISA plus immunoblotting) were tested by the ELISAs with the four purified recombinant virus proteins. On the basis of previous results showing a low background reading for porcine sera against the GST protein (antigen negative), the results of the recombinant protein-based ELISAs are presented as the ratio between the absorbance obtained for each serum sample against the GST-recombinant protein experimental preparations and the GST-negative control preparations. A cutoff value of 3 for the four recombinant protein-based ELISAs was established by comparing the ratios for 119 ASFV-negative and 80 ASFV-positive field serum samples.
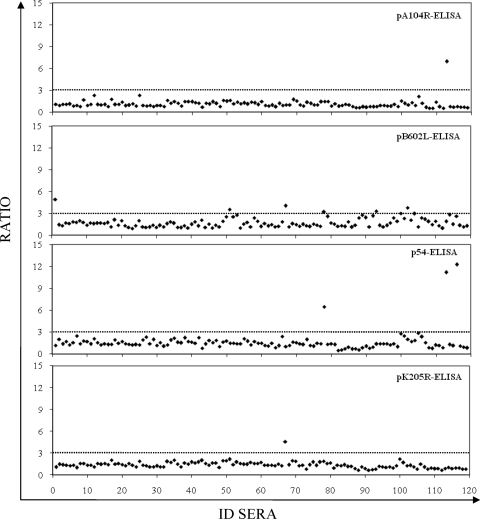
The numbers of serum samples among the 119 negative control serum samples tested with false-positive results were 1 each by the pA104R-based ELISA (pA104R-ELISA) and the pK205R-ELISA (99% specificity; 95% confidence interval [CI], 97.5 to 100), 3 by the p54-ELISA (97% specificity; 95% CI, 94.7 to 100), and 6 by the pB602L-ELISA (95% specificity; 95% CI; 91.0 to 98.9) (Fig. 2). A similar proportion of false-positive results was detected by the OIE-approved ELISA (6 of 119), resulting in a specificity of 95% (95% CI, 91.0 to 98.9).
FIG. 2.
Specificity of recombinant protein ELISAs. The 119 serum samples previously characterized as being ASFV negative by immunoblotting (OIE reference test) were tested by each recombinant protein ELISA. The ratios of the ODs obtained by each recombinant protein ELISA for the seronegative samples are shown. The dotted lines indicate the assay cutoff, which was established to be 3 for each recombinant protein ELISA. ID sera, serum sample number.
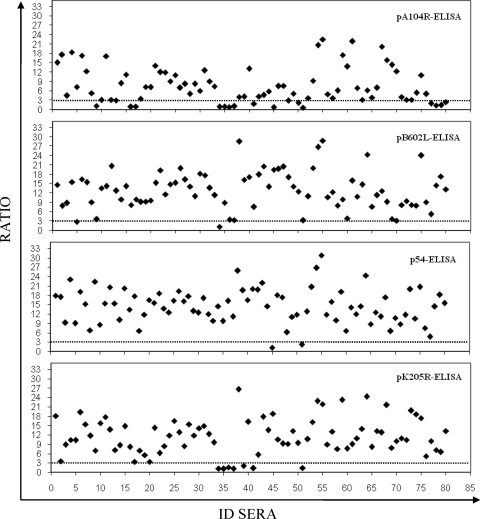
The numbers of false-negative serum samples detected among the 80 ASFV-positive serum samples were 17 by the pA104R-ELISA (79% sensitivity; 95% CI, 69.8 to 87.7), 7 by the pK205R-ELISA (91% sensitivity; 95% CI, 85.1 to 97.4), and 2 each by the p54- and pB602L-ELISAs (98% sensitivity; 95% CI, 94.1 to 100) (Fig. 3). By analysis of the seropositive samples by the OIE-approved ELISA, 75 of 80 serum samples were determined to be positive, achieving a sensitivity of 94% (95% CI, 88.4 to 99.1). The κ values showed a substantial to almost perfect agreement between the results of the reference test (immunoblotting) and the results of the pA104R-ELISA (κ = 0.81; 95% CI, 0.72 to 0.89) and almost perfect agreement between the results of the reference test and the results of the p54-ELISA (κ = 0.95; 95% CI, 0.90 to 0.99) and the pK205R-ELISA or the pB602L-ELISA (κ = 0.92; 95% CI, 0.86 to 0.97). Similar levels were observed for the OIE-ELISA (κ = 0.89; 95% CI, 0.82 to 0.95). A summary of the results obtained by each ELISA with the European field sera is shown in Table 1.
FIG. 3.
Sensitivity of recombinant protein ELISAs. The 80 serum samples previously characterized as being ASFV positive by immunoblotting (the OIE reference test) were analyzed by each recombinant protein ELISA. The ratios of the ODs obtained by each recombinant protein ELISA for the seropositive samples are shown. The dotted lines indicate the assay cutoff, which was established to be 3 for each recombinant protein ELISA. ID sera, serum sample number.
TABLE 1.
Performance of recombinant protein (pA104R, p54, pB602L, and pK205R)-based ELISAs compared to that of the conventional ELISA (OIE-ELISA)a
| Assay | % Sensitivity (95% CI) | % Specificity (95% CI) | κ (95% CI) |
|---|---|---|---|
| pA104R-ELISA | 79 (69.8-87.7) | 99 (97.5-100) | 0.81 (0.72-0.89) |
| p54-ELISA | 98 (94.1-100) | 97 (94.7-100) | 0.95 (0.90-0.99) |
| pK205R-ELISA | 91 (85.1-97.4) | 99 (97.5-100) | 0.92 (0.86-0.97) |
| pB602L-ELISA | 98 (94.1-100) | 95 (91.0-98.9) | 0.92 (0.86-0.97) |
| OIE-ELISA | 94 (88.4-99.1) | 95 (91.0-98.9) | 0.89 (0.82-0.95) |
A total of 199 serum samples (80 positive, 119 negative) from European domestic pigs that had previously been characterized by immunoblotting (OIE reference test) were analyzed.
Performance of recombinant protein-based ELISA with poorly preserved sera.
In the areas of Africa where ASFV is endemic, serum samples frequently reach the diagnostic laboratory after considerable time at ambient temperature and thus are in a degraded state. In order to assess the applicability of the new recombinant ELISAs to such sera, 39 field serum samples previously classified to be ASFV positive by OIE-approved tests were kept at 37°C for 1 month and were then tested by each recombinant protein-based ELISA. The results were compared with those obtained with the original untreated sera and are presented as a distribution chart in Fig. 4. As can be seen, there was a variable loss of reactivity, depending on which recombinant viral protein was used as the target of the ELISA. Although the heat-treated sera clearly functioned poorly by the pA104R-ELISA, most of the positive sera held for 1 month at 37°C remained positive by the p54- and pB602L-ELISAs. In fact, when the results obtained by the p54-, pB602L-, and pK205R-ELISAs are taken together, only 1 of the 39 heat-treated serum samples that was previously positive converted to having a negative result. The sensitivities after heat treatment were 95% (95% CI, 87.9 to 100), 87% (95% CI, 76.7 to 97.7), 80% (95% CI, 66.8 to 92.2), and 31% (95% CI, 16.3 to 45.3) for the p54-, pB602L-, pK205R-, and pA104R-ELISAs, respectively, whereas the sensitivity of the OIE-ELISA was 87% (95% CI, 76.7 to 97.7) (data not shown).
FIG. 4.
Sensitivity of recombinant protein ELISA after incubation of 39 porcine serum samples for 1 month at 37°C. Each bar corresponds to the value of the ratio for each serum sample. The dotted lines indicate the assay cutoff, which was established to be 3 for each recombinant protein ELISA. ID sera, serum sample number.
Performance of recombinant protein-based ELISAs with serum samples from Africa.
A total of 375 serum samples were obtained from domestic pigs in East Africa (Uganda, n = 254; Mozambique, n = 95) and West Africa (Nigeria, n = 6; Burkina Faso, n = 20), and 26 serum samples were collected from warthogs in Uganda. By taking the results of immunoblotting or the OIE-ELISA as the “gold standard,” most of the pig serum samples were negative, whereas all 26 warthog serum samples were positive (Table 2). The positive samples from West Africa (four from Nigeria, five from Burkina Faso) were positive by all four recombinant protein-based assays, and importantly, none of the four assays gave false-positive results for the negative sera from West Africa. Thus, with this limited number of serum samples, all four recombinant antigens were acceptable and reproducible recombinant antigen targets for the diagnosis of ASF with sera from West Africa.
TABLE 2.
Performance of recombinant protein (pA104R, p54, pB602L, and pK205R)-based ELISAs compared to that of the conventional ELISA (OIE-ELISA)a
| Country (serum sample source) | Performance of the following assayb:
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| OIE-ELISA
|
pA104R-ELISA
|
p54-ELISA
|
pK205R-ELISA
|
pB602L-ELISA
|
||||||
| Se | Sp | Se | Sp | Se | Sp | Se | Sp | Se | Sp | |
| Uganda (pigs) | 3/3 | 248/251 | 2/3 | 247/251 | 2/3 | 247/251 | 2/3 | 247/251 | 2/3 | 248/251 |
| Mozambique (pigs) | 4/4 | 85/91 | 1/4 | 88/91 | 3/4 | 90/91 | 3/4 | 83/91 | 3/4 | 90/91 |
| Nigeria (pigs) | 4/4 | 2/2 | 4/4 | 2/2 | 4/4 | 2/2 | 4/4 | 2/2 | 4/4 | 2/2 |
| Burkina Faso (pigs) | 5/5 | 15/15 | 5/5 | 15/15 | 5/5 | 15/15 | 5/5 | 15/15 | 5/5 | 15/15 |
| Uganda (warthogs) | 17/26 | 7/26 | 23/26 | 20/26 | 23/26 | |||||
A total of 375 serum samples obtained from domestic pigs in East Africa (Uganda, n = 254 [2 immunoblotting positive]; Mozambique, n = 95 [4 immunoblotting positive]) and West Africa (Nigeria, n = 6 [4 immunoblotting positive]; Burkina Faso, n = 20 [5 immunoblotting positive]) and 26 field serum samples obtained from warthogs in Uganda (all previously characterized as positive by immunoblotting) were analyzed.
Se, sensitivity; Sp, specificity. The data represent the number of serum samples positive (Se) or negative (Sp)/total number of serum samples tested.
Similarly, high specificities were obtained when the four recombinant viral proteins were tested with the sera from East Africa (Uganda and Mozambique): for the pA104R-ELISA, 98% specificity (95% CI, 96.5 to 99.5); for the p54-ELISA, 99% specificity (95% CI, 97.3 to 99.8); for the pK205R-ELISA, 96% specificity (95% CI, 94.5 to 98.4); for the pB602L-ELISA, 99% specificity (95% CI, 97.7 to 100); and for the OIE-ELISA, 97% specificity (95% CI, 95.7 to 99.1). Given the few serum samples from East Africa tested, there was no justification for calculation of the sensitivities, but with the frequency of positive results observed (specifically, the pA104R-, p54-, pK205R-, and pB602L-ELISAs revealed that two of three, two of three, two of three, and two of three of the serum samples from Uganda, respectively, were positive and that one of four, three of four, three of four, and three of four of the positive serum samples from Mozambique, respectively, were positive [Table 2]), we may conclude that these recombinant proteins are unlikely to provide acceptable sensitivities for the diagnosis of ASF with sera from East Africa. An explanation for this is not immediately obvious but is an urgent priority for the control of ASF in that part of Africa.
Strikingly, all 26 Ugandan warthogs tested positive by immunoblotting but only 17 of the 26 were positive by the OIE-ELISA. Analysis of these 26 warthog serum samples by the recombinant virus protein-based ELISAs was particularly interesting. A total of 23 of 26 were positive by the pB602L-ELISA and p54-ELISA, a marked improvement over the results of the OIE-ELISA (Table 2). In contrast, fewer positive serum samples (7 of 26) were detected by the pA104R-ELISA. Finally, the pK205R-ELISA was positive with 20 of 26 of the warthog serum samples.
Kinetics of anti-recombinant protein antibody responses in experimentally infected pig sera.
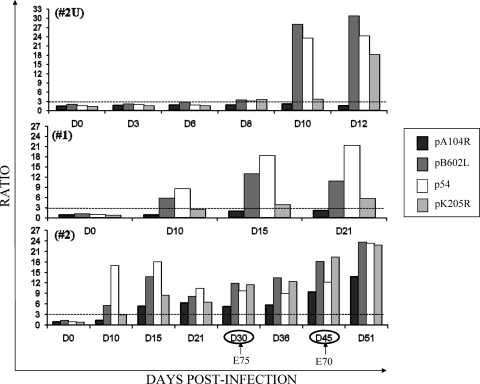
As low sensitivities were observed when sera from East African were assayed with the recombinant viral proteins, pigs 2U and 3U were experimentally infected with the virulent isolate Uganda ASFV Ug03H (Fig. 5). To provide a positive control, two pigs (pigs 1 and 2) were infected with the attenuated Spanish E75 CV14 isolate (Fig. 5). As expected, the pigs infected with the Spanish ASFV isolate did indeed make detectable antibodies to the recombinant proteins. Specifically, antibodies to the pB602L and p54 proteins were detected as early as 10 dpi in pigs 1 and 2. A similarly early antibody response was also observed when the same sera were tested by the OIE-ELISA against the total semipurifed ASFV antigen (data not shown). For pig 1, infected with the attenuated Spanish isolate, a positive, but low antibody response against pK205R was detected on days 15 and 21 postinfection, but antibodies to the pA104R protein were undetectable. The other pig (pig 2), infected with the same attenuated Spanish isolate, responded with antibodies against pK205R and pA104R by day 15 postinfection. Challenge of pig 2 at 30 dpi with homologous virulent Spanish isolate E75 did not appreciably raise the titers of antibodies against the four proteins. Reinoculation on day 45 of the same pig with heterologous virulent Spanish isolate E70 resulted in marginally increased antibody levels.
FIG. 5.
Detection of ASFV-specific antibodies from pigs experimentally infected with the Uganda ASFV Ug03H isolate (pig 2U) and the Spanish attenuated E75 CV14 isolate (pigs 1 and 2) by the recombinant protein ELISAs. The serum samples collected from pig 2U (upper graph), pig 1 (middle graph), and pig 2 (lower graph) at different times postinfection were analyzed by each recombinant protein ELISA. The arrows indicate the boosting of pig 2 with the virulent homologous E75 isolate and the heterologous E70 isolate. The dotted lines indicate the assay cutoff, which was established to be 3 for each recombinant protein ELISA.
Of the two pigs infected with virulent East Africa isolate Ug03H, one (pig 2U) responded with anti-pB602L and anti-pK205R antibody levels that were already above the cutoff line at day 8 postinfection, and in agreement with the results observed with pigs 1 and 2, the anti-p54 and anti-pB602L antibody levels were impressively high at 10 dpi. A similarly significant antibody response on day 10 was also observed when the same sera were tested by the OIE-ELISA (data not shown). The anti-pK205R antibody response showed a similar increase at day 12 postinfection. At this early time, as was observed with the Spanish E75 CV14 virus, no antibody against the pA104R protein was observed. The other pig infected with the Uganda isolate died on day 9 and had no detectable antibodies at day 8 postinfection.
DISCUSSION
In this study, we have assessed the use of four recombinant ASFV proteins (pA104R, pB602L, p54, and pK205R) as tools for a diagnostic ELISA for the serological diagnosis of ASF with samples of both European and African origin.
By analyzing European porcine field sera, ELISAs with recombinant proteins p54, pK205R, and pB602L performed well; and the results were in almost perfect agreement with those of the gold standard immunoblotting test for ASFV (κ values, 0.95 [95% CI, 0.90 to 0.99], 0.92 [95% CI, 0.86 to 0.97], and 0.92 [95% CI, 0.86 to 0.97], respectively). Thus, these ASFV recombinant proteins function as well as the OIE system, but without the necessity of working with infectious virus. Although the sensitivity of the pA104R-ELISA was low (79%; 95% CI, 69.8 to 87.7), it had the advantage of detecting only 1 sample with a false-positive result of the 119 control field serum samples tested, and so it may still be of value for the diagnosis of ASF when it is applied at the herd level.
In agreement with previous results with the p30 recombinant protein (22), both the OIE and the recombinant virus protein assays gave similar results with sera from Europe and Western Africa. Sera from Eastern African countries, however, gave variable results. The specificities of the four recombinant protein-based assays were very high and similar to the specificity of the OIE-ELISA. However, with the limited number of positive serum samples from Uganda (n = 3) and Mozambique (n = 4), the recombinant protein ELISAs (particularly the pA104R-ELISA) were less sensitive than the OIE-ELISA.
The decreased sensitivity with East African sera was evident and surprising. Clearly, the testing of more samples is required in order to make firm conclusions. As East African isolates are more variable and genotypically distant (5), the recombinant proteins were investigated for possible variations in their sequences. Bioinformatic analysis, however, showed that their sequences, and hence their immunological epitopes, are highly conserved. In view of the structural conservation of the four proteins that we have studied, an explanation for their unexpectedly low sensitivities with sera from East Africa may reside in the pig rather than the virus; for example, the pigs may have different immunological repertoires, clonal deletions, swine leukocyte antigen types, and balances of innate immune mechanisms. Surprisingly, the pA104R protein, although it is highly conserved in viruses isolated from pigs, warthogs, and ticks from Europe and East and West Africa, was the least sensitive of the four proteins with sera from East Africa.
A similar analysis of sera collected from warthogs was particularly interesting and highly relevant for the control of sporadic outbreaks in domestic pigs in contact with warthogs, raising the possibility of the performance of proactive serosurveillance of both domestic and wild pigs in order to determine the status of ASF in East African countries when the sylvatic cycle plays a crucial part in the epidemiology of the disease. Although the number of warthog serum samples was small, the comparative serology results obtained by the recombinant protein-based ELISAs and the OIE-ELISA strongly suggest that the pB602L- and p54-based ELISAs are more sensitive than the OIE-ELISA or the ELISA with well-known antigenic protein p30 (22).
In addition to the high sensitivities and specificities, antibodies against proteins p54 and pB602L were detectable in sera as early as 10 dpi following experimental infection of pigs with either Ugandan and Spanish isolates (similar results were obtained when whole virus was used as the antigen). The antibody titers were maintained for up to 30 dpi (the last time point at which samples were obtained) after infection with the Spanish E75 CV14 virus isolate. After the boost with the homologous virulent isolate (isolate E75), the antibody response against all the proteins tested was slightly increased 15 days after the boost. Boosting with heterologous virulent isolate E70, however, resulted in a more significant increase in the antibody response.
A particular disadvantage of the conventional ELISA (which uses semipurified virions as the antigen) is the lack of sensitivity and the unacceptable level of false-negative results obtained with poorly preserved samples, hence demanding the routine confirmation of the result by immunoblotting (2, 3, 20). In order to evaluate the stability of the new ELISAs, 39 ASFV-positive field serum samples were incubated for 1 month at 37°C in order to simulate deterioration in the field and were then retested by the ELISA with the recombinant proteins. With these sera, a sensitivity of 95% (95% CI, 87.9 to 100) was obtained with protein p54, whereas a sensitivity of 87% (95% CI, 76.7 to 97.7) was obtained by the OIE-ELISA. Only the pA104R-ELISA gave a major loss in sensitivity, which was reduced to 31% (95% CI, 16.3 to 45.3). Thus, the best candidates for the serodiagnosis of ASF are the p54 and pB602L proteins, although the pB602L protein had a marginally diminished sensitivity with the heat-treated sera. Importantly, taking together the results obtained with three proteins, p54, pB602L, and pK205R, only 1 of the 39 heat-treated serum samples was negative, and so sensitive diagnosis with recombinant viral proteins is possible even with poorly preserved sera.
It is not clear why the same sera should lose reactivity against one recombinant protein but not another. Two possibilities, proteolytic degradation of the recombinant antigen by heated sera and differential representation of porcine IgG classes, have been eliminated (our unpublished work). A possible explanation for this could be that the antibodies against the p54 protein are more stable or display a higher affinity than the other proteins, and this allows them to react even after the long incubation at 37°C.
In conclusion, this work extends the recombinant antigens (2, 9, 19, 22) with the potential for use for the diagnosis of ASFV infections, has confirmed the usefulness of p54, and has identified pB602L as a novel diagnostic tool for use in areas where ASF is endemic. Given that the variability of ASFV isolates in Africa is greater than that in Europe, further studies are needed in African countries in order to adequately validate the ELISAs. Work is in progress to address this issue by using sera collected from East Africa and Eastern European countries where recent outbreaks of ASF have occurred.
Acknowledgments
This work was supported by European Union project ASFRISK grant agreement 211691 and Wellcome Trust project WT075813MA. Work at INIA was supported by the Community Reference Laboratory for ASF (grant UE-LR PPA/03) and Spanish grants from MEC (grant ILRI2003-001). A. L. Reis was a recipient of a fellowship (SFRH/BD/6071/2001) from the Fundação para a Ciência e a Tecnologia.
We thank E. Martin and A. Simon for their valuable technical assistance.
Footnotes
Published ahead of print on 6 May 2009.
REFERENCES
- 1.Alcaraz, C., M. De Diego, M. J. Pastor, and J. Escribano. 1990. Comparison of a radioimmunoprecipitation assay to immunoblotting and ELISA for detection of antibody to African swine fever virus. J. Vet. Diagn. Investig. 2191-196. [DOI] [PubMed] [Google Scholar]
- 2.Alcaraz, C., F. Rodriguez, J. M. Oviedo, A. Eiras, M. De Diego, C. Alonso, and J. M. Escribano. 1995. Highly specific confirmatory Western blot test of African swine fever virus antibody detection using the recombinant virus protein p54. J. Virol. Methods 52111-119. [DOI] [PubMed] [Google Scholar]
- 3.Arias, M., J. M. Escribano, and J. M. Sanchez-Vizcaino. 1993. Persistence of African swine fever antibody reactivity on ELISA and immunoblotting assays. Vet. Rec. 133189. [DOI] [PubMed] [Google Scholar]
- 4.Bech-Nielsen, S., M. L. Arias, J. Panadero, J. M. Escribano, C. Gomez Tejedor, Q. Perez-Bonilla, and J. M. Sánchez Vizcaíno. 1993. Laboratory diagnosis and disease occurrence in the current African swine fever eradication program in Spain 1989-1991. Prev. Vet. Med. 17225-234. [Google Scholar]
- 5.Boshoff, C. I., A. D. Bastos, L. J. Gerber, and W. Vosloo. 2007. Genetic characterisation of African swine fever viruses from outbreaks in southern Africa (1973-1999). Vet. Microbiol. 12145-55. [DOI] [PubMed] [Google Scholar]
- 6.Dixon, L. K., J. V. Costa, J. M. Escribano, D. L. Rock, E. Vinuela, and P. J. Wilkinson. 2000. Asfarviridae, p. 159-165. In M. H. V van Regenmortel, C. M. Fauquet, D. H. L. Bishop, E. Cartens, M. Estes, S. Lemon, J. Maniloff, M. A. Mayo, D. McGeoch, C. R. Pringle, and R. B. Wickner (ed.), Virus taxonomy. Seventh Report of the International Committee on Taxonomy of Viruses. Academic Press, San Diego, CA.
- 7.Escribano, J. M., M. J. Pastor, and J. M. Sánchez Vizcaíno. 1989. Antibodies to bovine serum albumin in swine sera: implication for false-positive reactions in the serodiagnosis of African swine fever. Am. J. Vet. Res. 501118-1122. [PubMed] [Google Scholar]
- 8.Everitt, B. S. 1989. Statistical methods for medical Investig. Oxford University Press, London, United Kingdom.
- 9.Gallardo, C., E. Blanco, J. M. Rodriguez, A. L. Carrascosa, and J. M. Sanchez Vizcaino. 2006. Antigenic properties and diagnostic potential of African swine fever virus protein pp62 expressed in insect cells. J. Clin. Microbiol. 44950-956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gomez-Puertas, P., F. Rodriguez, J. M. Oviedo, A. Brun, C. Alonso, and J. M. Escribano. 1998. The African swine fever virus proteins p54 and p30 are involved in two distinct steps of virus attachment and both contribute to the antibody-mediated protective immune response. Virology 243461-471. [DOI] [PubMed] [Google Scholar]
- 11.Hess, W. R. 1971. African swine fever virus, p. 1-3. In S. Gad, G. Hallaner, and K. F. Meyer (ed.), Virology monographs, vol. 9. Springer, Berlin, Germany. [DOI] [PubMed] [Google Scholar]
- 12.Kollnberger, S. D., B. Gutierrez-Castañeda, M. Foster-Cuevas, A. Corteyn, and R. M. Parkhouse. 2002. Identification of the principal serological immunodeterminants of African swine fever virus by screening a virus cDNA library with antibody. J. Gen. Virol. 831331-1342. [DOI] [PubMed] [Google Scholar]
- 13.Leitão, A., C. Cartaxeiro, R. Coelho, B. Cruz, R. M. Parkhouse, F. Portugal, J. D. Vigario, and C. L. Martins. 2001. The non-haemadsorbing African swine fever virus isolate ASFV/NH/P68 provides a model for defining the protective anti-virus immune response. J. Gen. Virol. 82513-523. [DOI] [PubMed] [Google Scholar]
- 14.Malmquist, W., and D. Hay. 1960. Hemadsorption and cytopathic effect produced by African swine fever virus in swine bone marrow and buffy coat cultures. Am. J. Vet. Res. 21104-108. [PubMed] [Google Scholar]
- 15.Neilan, J. G., L. Zsak, Z. Lu, T. G. Burrage, G. F. Kutish, and D. L. Rock. 2004. Neutralizing antibodies to African swine fever virus proteins p30, p54, and p72 are not sufficient for antibody-mediated protection. Virology 319337-342. [DOI] [PubMed] [Google Scholar]
- 16.Office International des Epizooties. July 2008, posting date. African swine fever. In Manual of diagnostic tests and vaccines for terrestrial animals 2008. Office International des Epizooties, Paris, France. http://www.oie.int/eng/normes/mmanual/2008/pdf/2.08.01_ASF.pdf.
- 17.Onisk, D. V., M. V. Borca, G. Kutish, E. Kramer, P. Irusta, and D. L. Rock. 1994. Passively transferred African swine fever virus antibodies protect swine against lethal infection. Virology 198350-354. [DOI] [PubMed] [Google Scholar]
- 18.Oura, C. A., M. S. Denyer, H. Takamatsu, and R. M. Parkhouse. 2005. In vivo depletion of CD8+ T lymphocytes abrogates protective immunity to African swine fever virus. J. Gen. Virol. 862445-2450. [DOI] [PubMed] [Google Scholar]
- 19.Oviedo, J. M., F. Rodriguez, P. Gomez-Puertas, A. Brun, N. Gomez, C. Alonso, and J. M. Escribano. 1997. High level expression of the major antigenic African swine fever virus proteins p54 and p30 in baculovirus and their potential use as diagnosis reagents. J. Virol. Methods 6427-35. [DOI] [PubMed] [Google Scholar]
- 20.Pastor, M. J., M. D. Laviada, J. M. Sanchez-Vizcaino, and J. M. Escribano. 1989. Detection of African swine fever virus antibodies by immunoblotting assay. Can. J. Vet. Res. 53105. [PMC free article] [PubMed] [Google Scholar]
- 21.Pastor, M. J., M. Arias, and J. M. Escribano. 1990. Comparison of two antigens for use in an enzyme-linked immunosorbent assay to detect African swine fever antibody. Am. J. Vet. Res. 511540-1543. [PubMed] [Google Scholar]
- 22.Perez-Filgueira, D. M., F. Gonzalez-Camacho, C. Gallardo, P. Resino-Talavan, E. Blanco, E. Gomez-Casado, C. Alonso, and J. M. Escribano. 2006. Optimization and validation of recombinant serological tests for African swine fever diagnosis based on detection of the p30 protein produced in Trichoplusia ni larvae. J. Clin. Microbiol. 443114-3121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Reis, A. L., R. M. Parkhouse, A. R. Penedos, C. Martins, and A. Leitao. 2007. Systematic analysis of longitudinal serological responses of pigs infected experimentally with African swine fever virus. J. Gen. Virol. 882426-2434. [DOI] [PubMed] [Google Scholar]
- 24.Ruiz-Gonzalvo, F., M. E. Carnero, C. Caballero, and J. Martínez. 1986. Inhibition of African swine fever infection in the presence of immune sera in vivo and in vitro. Am. J. Vet. Res. 471249-1252. [PubMed] [Google Scholar]
- 25.Schlafer, D. H., J. W. McVicar, and C. A. Mebus. 1984. African swine fever convalescent sows: subsequent pregnancy and the effect of colostral antibody on challenge inoculation of their pigs. Am. J. Vet. Res. 451361-1366. [PubMed] [Google Scholar]
- 26.Wardley, R. C., S. G. Norley, P. J. Wilkinson, and S. Williams. 1985. The role of antibody in protection against African swine fever virus. Vet. Immunol. Immunopathol. 9201-212. [DOI] [PubMed] [Google Scholar]
- 27.Yanez, R. J., J. M. Rodriguez, M. L. Nogel, L. Yuste, C. Enriquez, J. F. Rodriguez, and E. Vinuela. 1995. Analysis of the complete nucleotide sequence of African swine fever virus. Virology 208249-278. [DOI] [PubMed] [Google Scholar]