Abstract
Entamoeba histolytica is a protozoan parasite that causes amoebic dysentery and liver abscess. Vesicle trafficking events, such as phagocytosis and delivery of plasma membrane proteins, have been implicated in pathogenicity. Rab GTPases are proteins whose primary function is to regulate vesicle trafficking; therefore, understanding the function of Rabs in this organism may provide insight into virulence. E. histolytica possesses a number of unique Rabs that exhibit limited homology to host Rabs. In this study we examined the function of one such Rab, EhRabA, by characterizing a mutant overexpressing a constitutively GTP-bound version of the protein. Overexpression of mutant EhRabA resulted in decreased adhesion to and phagocytosis of human red blood cells and in the appearance of large tubular organelles that could be stained with endoplasmic reticulum (ER)-specific but not Golgi complex-specific antibodies. Consistent with the adhesion defect, two subunits of a cell surface adhesin, the galactose/N-acetylgalactosamine lectin, were mislocalized to the novel organelle. A cysteine protease, EhCP2, was also localized to the ER-like compartment in the mutant; however, the localization of two additional cell surface proteins, Igl and SREHP, remained unchanged in the mutant. The phenotype of the mutant could be recapitulated by treatment with brefeldin A, a cellular toxin that disrupts ER-to-Golgi apparatus vesicle traffic. This suggests that EhRabA influences vesicle trafficking pathways that are also sensitive to brefeldin A. Together, the data indicate that EhRabA directly or indirectly influences the morphology of secretory organelles and regulates trafficking of a subset of secretory proteins in E. histolytica.
Entamoeba histolytica, a protozoan parasite, is the causative agent of amoebic dysentery and causes liver abscess. It is prevalent in developing countries that cannot prevent its fecal-oral spread and is responsible for considerable global morbidity and mortality (reviewed in reference 12). Infection is acquired by ingestion of the cyst form of the parasite. Excystation occurs in the small intestine, and released amoeboid trophozoites move to, and colonize, the bowel lumen. Here, the pathogen acquires nutrients via phagocytosis of colonic bacteria, host cells, and host cell debris. The ability of the pathogen to carry out phagocytosis has been correlated to its virulence potential. For example, phagocytosis-deficient mutants of E. histolytica exhibit reduced pathogenicity in vitro and in vivo (24, 33), and a noninvasive Entamoeba species, E. dispar, exhibits low rates of phagocytosis (26).
Given the importance of phagocytosis to virulence, the identification of proteins that directly or indirectly regulate this process has been the focus of a considerable research effort (reviewed in references 16 and 23). Proteomic analyses of purified phagosomes have identified numerous proteins that may regulate phagocytosis in E. histolytica (2, 17, 22). Functional studies have identified several cell surface proteins as possible phagocytic receptors. One such receptor, the galactose/N-acetylgalactosamine-inhibitable lectin (Gal/GalNAc lectin) (6, 31), consists of a transmembrane heavy subunit (Hgl) which is covalently associated with a glycosylphosphatidylinositol (GPI)-linked light subunit (Lgl). This heterodimer is noncovalently associated with a GPI-anchored intermediate subunit (Igl). Other cell surface receptors include SREHP, a serine-rich protein that is proposed to be lipid anchored (42), and PATMK, a transmembrane kinase (2).
The complement of receptors found on the cell surface is influenced both by anterograde and retrograde vesicle trafficking pathways. For example, newly synthesized proteins are incorporated into the membrane of the endoplasmic reticulum (ER), transported to the Golgi apparatus, and finally delivered to the plasma membrane via anterograde transport vesicles. On the other hand, cell surface receptors may be internalized and delivered to intracellular compartments or back to the plasma membrane via retrograde transport vesicles (44). While E. histolytica trophozoites have been shown to possess an ER and Golgi apparatus (8, 19, 41), insight into the molecular mechanisms regulating the morphological and functional integrity of these organelles is limited. Such insight would contribute to our understanding of phagocytosis, which relies on the proper functioning of these organelles for localization of cell surface receptors.
In other systems, secretory vesicle trafficking events are regulated by Rab GTPases, a family of GTP-binding proteins involved in the docking and fusion of transport vesicles with target membranes. Substantial evidence suggests that Rab proteins carry out their function by cycling between a GDP-bound cytosolic form and a GTP-bound membrane form (reviewed in reference 39). In silico genome mining has shown that E. histolytica possesses greater than 90 Rab GTPases (34). A number of these exhibit limited homology to other known Rabs and thus are considered unique to E. histolytica (34). We previously reported that one of these unique Rabs, EhRabA, may be involved in the regulation of polarization, motility, and actin cytoskeletal dynamics (47, 48). In the current study, we demonstrate that overexpression of the putatively GTP-bound form of EhRabA results in the alteration of ER morphology, mislocalization of two subunits of the Gal/GalNAc lectin, and reduced phagocytosis, suggesting that this Rab plays a direct or indirect role in cellular functions that contribute to virulence.
MATERIALS AND METHODS
Strains, culture, and assay conditions.
E. histolytica trophozoites (strain HM-1:IMSS) were cultured axenically in TYI-S-33 medium (7) in 15-ml glass screw cap tubes at 37°C. Several assays were performed in serum-free E. histolytica medium, TYI-33. Chinese hamster ovary (CHO) cells were grown at 37°C in Dulbecco's modified Eagle's medium supplemented with fetal bovine serum (10%, vol/vol), 100 U/ml penicillin-streptomycin, and 1 mM HEPES.
Antibodies.
Specific antibodies recognizing the subunits of the Gal/GalNAc lectin were gifts of W. A. Petri, Jr. (University of Virginia, Charlottesville). The generation of specific antibodies recognizing EhRabA, calreticulin (gift of N. Guillén, Unité de Biologie Cellulaire du Parasitisme, Institut Pasteur, Paris, France), EhCP2, and SREHP (gifts of S. L. Stanley, Jr., Washington University School of Medicine, St. Louis, MO) has been described elsewhere (9, 38, 48, 50). Monoclonal and polyclonal antibodies recognizing the hemagglutinin (HA) epitope were obtained from Roche Pharmaceuticals (Nutley, NJ) and Zymed (South San Francisco, CA), respectively. The 10C3 mouse monoclonal antibody recognizing the KDEL ER retention signal was obtained from Abcam (Cambridge, MA).
Western blot analysis.
Western blot analysis of E. histolytica cell lysates was performed as described previously (47). Dilutions for anti-EhRabA, anti-Hgl, anti-Igl, anti-KDEL, or anti-SREHP primary antibodies were 1:1500, 1:5,000, 1:2,500, 1:250, and 1:800, respectively. Commercial polyclonal anti-HA antibody was utilized at 2 μg/ml. In all cases, Western blots were normalized by loading equal cell number equivalents to each of the lanes.
Mutagenesis of the EhRabA cDNA and transfection of E. histolytica trophozoites.
Mutagenesis was carried out by a PCR-based method to generate a constitutively GTP-bound version of EhRabA (EhRabAQ84L). In particular, the codon for glutamine (Q; CAA), at amino acid position 84, was changed to the codon (CTA) for leucine (L) by using the QuikChange kit (Stratagene, La Jolla, CA) according to the manufacturer's protocol. Nucleotides encoding an N-terminal HA tag, a 5′-BamHI site, and 3′-SalI site were also incorporated into the cDNA by PCR as described previously (47). The resulting PCR product was digested with BamHI and SalI and ligated into the E. histolytica expression vector pGIR209 (30) (gift of W. A. Petri, Jr., University of Virginia, Charlottesville), which had been digested with BglII and SalI. This vector allows for the inducible expression of exogenous proteins by the addition of tetracycline to the medium and is cotransfected with a second vector, pGIR308, that encodes the tetracycline repressor (30).
Exponentially growing trophozoites of E. histolytica were transfected with the engineered vector as described previously (43). The transfected amoebae were maintained in 6 μg/ml G418 (for pGIR209) and 15 μg/ml hygromycin (for pGIR308), which were added to the medium. Mutant EhRabA (the “induced mutant”) expression was induced by the addition of 5 μg/ml tetracycline to the culture medium for 24 h.
Measurement of phagocytosis.
Uptake of human red blood cells (hRBCs; U.S. Biological Corp., Swampscott, MA) was measured according to the methods of Voigt et al. (45). Pretreatment of erythrocytes with calcium and the measurement of uptake of calcium-treated hRBCs were carried out according to the methods of Boettner et al. (3).
IF microscopy.
For immunofluorescence (IF) microscopy, wild-type or mutant E. histolytica trophozoites were fixed with 4% (vol/vol) paraformaldehyde in phosphate-buffered saline (15 min, room temperature [RT]). Following permeabilization with 0.2% (vol/vol) Triton X-100 in PBS (5 min, RT), nonspecific binding sites were blocked by incubation with 3% bovine serum albumin-10% goat serum-PBS (30 min, RT). The amoebae were then incubated with the following dilutions of primary antibodies for 2 h at room temperature: polyclonal anti-Hgl (1:500), monoclonal anti-Hgl (1:66), anti-Igl (1:100), anti-Lgl (1:66), anti-KDEL (1:140), anti-calreticulin (1:100), anti-SREHP (1:100), anti-EhCP2 (1:100), or monoclonal anti-HA (1:83). In some cases, wild-type trophozoites were treated with brefeldin A (BFA; 100 μg/ml, 4 h, 37°C) prior to staining with primary antibodies.
Following primary antibody incubation, and where appropriate, the amoebae were incubated with one of the following secondary antibodies: Alexa Fluor 488 (green) or Alexa Fluor 594 (red) conjugated to goat anti-mouse or anti-rabbit immunoglobulin G (diluted 1:1,000 in 1% bovine serum albumin-phosphate-buffered saline; Invitrogen, Carlsbad, CA; 1 h, RT). In some instances, to stain nuclei, propidium iodide (20 μg/ml) was added for 10 min during secondary antibody incubation. Stained trophozoites were mounted in SlowFade (Invitrogen) and observed using a Carl Zeiss (Thornwood, NY) LSM 510 laser scanning confocal microscope.
Measurement of adhesion to erythrocytes.
Adhesion of E. histolytica amoebae to erythrocytes was assayed using a previously described rosette formation assay (31). Briefly, trophozoites (1 × 105) and erythrocytes (1 × 106) were mixed in 500 μl of TYI-33, centrifuged (200 × g, 4°C, 5 min), and incubated for 30 min on ice. Following incubation, 450 μl of the supernatant was removed and 50 μl trypan blue was added. Ten microliters of the resulting mixture was used for counting on a hemacytometer. Adherent amoebae were defined as those with three or more bound erythrocytes (31). In some instances, trophozoites were pretreated with 55 mM galactose or 55 mM mannose for 30 min prior to exposure to erythrocytes and were maintained in the presence of these sugars during exposure to erythrocytes.
Measurement of adhesion to host monolayers.
Measurement of adhesion of trophozoites to monolayers of CHO cells was carried out as described previously (29).
Statistical analyses.
All values are given as means ± standard deviations (SD). Statistical analyses were performed using GraphPad Instat V.3 with an unpaired t test, Welch corrected (two-tail P value). P values of less than 0.05 were considered statistically significant. P values less than 0.01 or 0.001 were considered highly statistically significant.
RESULTS
Generation of an E. histolytica mutant expressing a GTP-bound form of EhRabA.
To gain insight into the function of EhRabA we generated an E. histolytica cell line that conditionally overexpressed a constitutively GTP-bound version of this protein. The cDNA encoding EhRabA was modified in two ways. First, the cDNA was mutagenized to change the codon for glutamine (Q) at amino acid position 84 to that for leucine (L), to generate a mutant version termed EhRabAQ84L. This mutation was chosen based on an identical mutation made in numerous other small-molecular-weight GTPases (5, 28, 35), including a Rab5-like GTPase of E. histolytica (35). In other systems, this mutation has been shown to dramatically slow GTPase activity resulting in Rabs that are constitutively GTP bound (40). Second, to distinguish mutant EhRabA from endogenous EhRabA, the 5′ end of the EhRabA cDNA was modified to include nucleotides encoding the HA epitope (4). The N-terminal fusion was chosen to avoid interference with C-terminal structural elements responsible for membrane association of proteins belonging to the Ras superfamily (36).
The modified cDNA was inserted into the E. histolytica expression vector, pGIR209, which confers G418 (neomycin) resistance and allows for tetracycline-inducible expression of exogenous genes when introduced into trophozoites (30). A standard electroporation protocol (43) was utilized to introduce the expression vector into trophozoites which had been previously transfected with an additional plasmid, pGIR308. This companion plasmid encodes the tetracycline repressor protein, which is necessary for tetracycline inducibility. Transfection was confirmed by purification of the expression plasmid from stably transfected cell lines as described previously (43) and sequencing of the cDNA insert (data not shown).
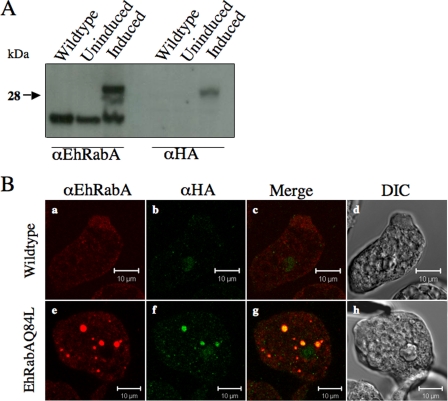
Maximum expression of modified EhRabA was achieved by the addition of 5 μg/ml tetracycline to the culture medium for 24 h. Western blot analysis using anti-EhRabA antibody revealed two forms of the EhRabA protein in transformed cells after induction with tetracycline: a lower-molecular-mass species (23 kDa) representing endogenous EhRabA, and a higher-molecular-mass species (29 kDa) representing the HA-tagged mutant version (Fig. 1A). The latter exhibited a molecular mass that was slightly higher than expected; however, we cannot rule out the possibility that the mutation alters posttranslational modifications. Decreased prenylation and/or increased methylation, two modifications normally seen on Rabs (13, 37), would have resulted in a slower electrophoretic mobility.
FIG. 1.
Expression of endogenous and mutant EhRabA in cell lysates from wild-type cells or transformed cells before (uninduced mutant) and after (induced mutant) induction with tetracycline for 24 h. (A) Cell lysates from wild-type, uninduced, and induced mutant cell lines were resolved by SDS-PAGE and protein was detected by Western blotting using antibodies specific for EhRabA or to the HA epitope. (B) IF microscopy of wild-type (a to c) and mutant (e to g) cells using anti-EhRabA and anti-HA antibodies. Colocalized antigens are shown in yellow (c and g), and corresponding differential interference contrast microscopic images are provided (d and h). In wild-type cells, EhRabA localizes to small cytoplasmic puncta (a), which do not colocalize with nonspecific HA-staining (b and c). In mutant cells, EhRabA localizes to small and large cytoplasmic puncta. A fraction of EhRabA-positive puncta colocalize with the HA epitope (f and g). Bars, 10 μm.
An intermediate-molecular-mass protein was also detected in the mutants by Western blotting with EhRabA-specific antibody. This protein species may represent a degradation product of the higher-molecular-mass species, as it was not recognized by HA-specific antibody (Fig. 1A). The HA-tagged protein was not visible in lysates of wild-type cells or in lysates of mutant cells prior to tetracycline induction (Fig. 1A), confirming the inducibility of the expression system. Longer inductions with tetracycline (48 to 72 h) did not result in higher levels of the mutant protein as determined by Western blot analysis (data not shown). Therefore, for all subsequent studies, expression of mutant EhRabA was induced by the addition of tetracycline 24 h prior to experimentation.
In a previous study we demonstrated that EhRabA localized to small vesicles in the cytoplasm or in extending membranes of motile cells (48). IF staining using antibodies specific for EhRabA or for the HA epitope demonstrated that EhRabAQ84L also localized to both small and large puncta throughout the cytoplasm (Fig. 1B). The mutant protein was rarely observed to accumulate in membrane extensions.
Expression of GTP-bound EhRabA reduces phagocytosis.
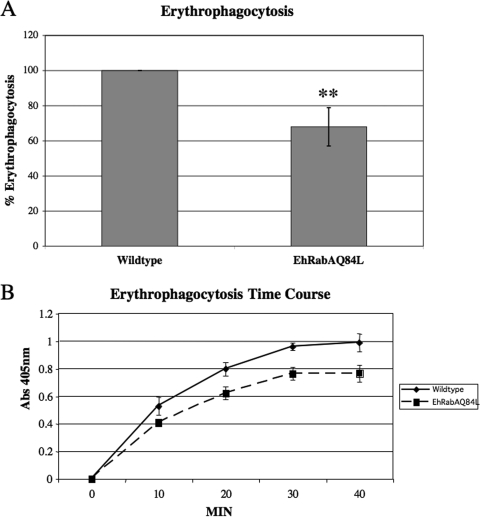
Previously, EhRabA was shown to localize to cellular extensions in the leading edge of motile trophozoites (48). Since extension of pseudopodia is required for some forms of phagocytosis, and since phagocytosis represents an important virulence function of E. histolytica, we measured uptake of hRBCs in mutants expressing EhRabAQ84L. Wild-type and mutant trophozoites were exposed to hRBCs for 10 min, after which uptake of heme was quantified spectrophotometrically. Expression of EhRabAQ84L resulted in a statistically significant reduction in erythrophagocytosis (Fig. 2A).
FIG. 2.
Cells expressing EhRabAQ84L exhibit reduced erythrophagocytosis. (A) Wild-type cells and cells expressing EhRabAQ84L were exposed to erythrocytes for 10 min, after which the level of ingested heme was measured by spectrophotometry. The data are reported as the percentage of uptake by wild-type cells, which was arbitrarily set to 100%. Uptake by the mutants was significantly lower than that of wild-type cells. **, P < 0.01. The data represent the means ± SD of four trials. (B) Wild-type cells and cells expressing EhRabAQ84L were exposed to erythrocytes for 40 min. At the indicated times, trophozoites were collected by centrifugation and the level of ingested heme was measured by spectrophotometry. The data are reported as means ± SD of absorbance at 405 nm for four trials. While mutant cells took up less heme overall, both cell lines exhibited similar kinetics of uptake.
To gain further insight into phagosomal trafficking in the engineered cell line, we exposed wild-type and mutant trophozoites to hRBCs for time periods of up to 40 min. At regular intervals, samples of trophozoites were taken and the level of internalized heme was quantified. Although the cell line expressing EhRabAQ84L internalized less heme overall, both wild-type and mutant cells exhibited an increase in heme accumulation up to 30 min, after which steady-state kinetics were apparent (Fig. 2B). That both cell lines displayed similar kinetics suggests that intracellular trafficking or maturation of phagosomes may not be altered in the mutant. Rather, the reduction in hRBC uptake by the engineered cell line may be the result of a defect in an early stage(s) of erythrophagocytosis, such as adhesion to the target.
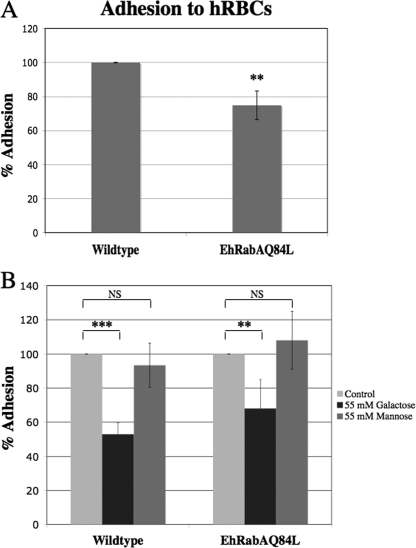
Expression of GTP-bound EhRabA alters galactose-sensitive adhesion to hRBCs.
To specifically examine early erythophagosomal events in the mutants, we measured their ability to adhere to erythrocytes by using a standard rosette assay (31). Mutant cells were less efficient at binding hRBCs than wild-type cells (Fig. 3A), suggesting that this early erythrophagosomal event was inhibited by expression of EhRabAQ84L. Since E. histolytica trophozoites rely, in part, on the Gal/ GalNAc lectin for adhesion to hRBCs (31), we also determined whether galactose or mannose (negative control) could inhibit the interaction between mutant cells and hRBCs. In wild-type cells, galactose was capable of reducing adhesion to hRBCs by approximately 47%, whereas in mutant cells, the same concentration of galactose inhibited adhesion to hRBCs by 32% (Fig. 3B). Reduced sensitivity of the mutant to galactose inhibition suggests decreased functionality for the Gal/GalNAc lectin in these cells. Mannose was incapable of inhibiting parasite-hRBC interactions for either cell line (Fig. 3B), indicating that the observed inhibition of adhesion by galactose was authentic.
FIG. 3.
Mutant cells exhibit reduced adhesion to hRBCs. The ability of wild-type and mutant cells to adhere to hRBCs was determined by a standard rosette assay in the absence (control) and presence of galactose or mannose. (A) Mutant cells were less efficient at binding hRBCs compared to wild-type cells. **, P < 0.01. (B) While incubation in the presence of galactose significantly reduced adhesion of both cell lines to hRBC targets, galactose was less efficient at inhibiting adhesion of mutant cells compared to wild-type cells. *** P < 0.001; **, P < 0.01. Mannose did not significantly (i.e., nonsignificantly [NS]) reduce adhesion of either cell line to host cell targets, indicating that galactose-mediated inhibition of adhesion was authentic. For both panels A and B, the data represent the means ± SD of five trials and are reported as a percentage of adhesion of control cells to hRBCs, which was arbitrarily set to 100%.
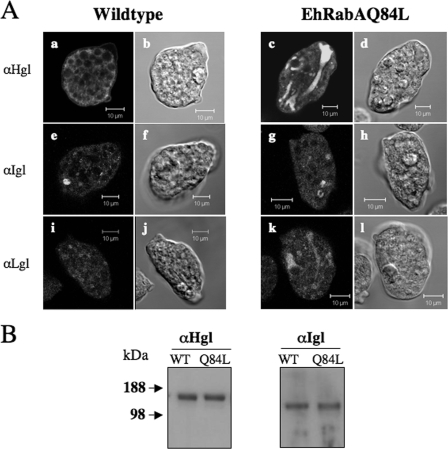
Expression of GTP-bound EhRabA alters the subcellular localization but not levels of subunits of the Gal/GalNAc lectin.
The finding that cells expressing EhRabAQ84L were less sensitive to galactose inhibition of adhesion provided the impetus to examine the subcellular localization and the level of Gal/GalNAc lectin subunits in these cells. IF microscopy demonstrated that, in mutant cells, Hgl was minimally localized to the plasma membrane but was found to be enriched in large tubular intracellular compartments (Fig. 4A, panel c). This differed from the pattern observed in wild-type cells, where Hgl was localized mainly to the cell periphery and to puncta throughout the cytosol (Fig. 4A, panel a). Since Hgl possesses the ligand binding site, the altered localization of this subunit in the mutants may lead to their reduced ability to bind hRBCs.
FIG. 4.
Subcellular localization of the Gal/GalNAc subunits in wild-type cells and cells expressing EhRabAQ84L. (A) IF microscopy of wild-type (a, e, and i) and mutant cells (c, g, and k) using anti-Hgl, anti-Igl, or anti-Lgl antibodies. Corresponding differential interference contrast microscopic images are shown (b, d, f, h, j, and l). In wild-type cells, Hgl, Igl, and Lgl are found in puncta throughout the cytoplasm. In wild-type cells, Hgl is also enriched at the plasma membrane. In mutant cells, Hgl and Lgl are localized to intracellular perinuclear compartments, whereas Igl is localized to cytoplasmic puncta. Bars, 10 μm. (B) Western blot analysis of trophozoite lysates from wild-type (WT) and mutant (Q84L) cells performed with anti-Hgl or anti-Igl antibody.
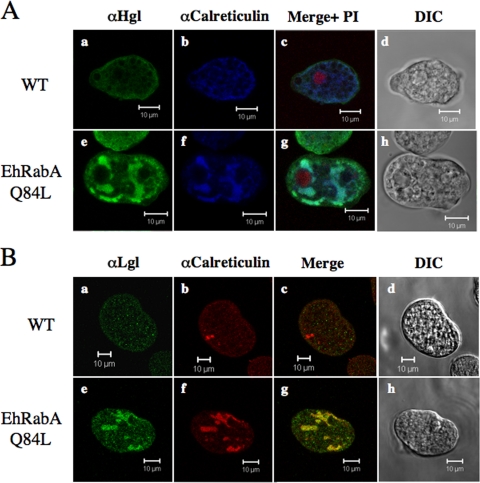
We also examined the subcellular localizations of the other subunits of the Gal/GalNAc lectin. In the mutant cells, Lgl exhibited a similar localization to that of Hgl, concentrating in an intracellular tubular network (Fig. 4A, panel k). Lgl is covalently attached to Hgl through disulfide bridges (14); therefore, it was not surprising to observe an altered localization of Lgl. Interestingly, the distribution of Igl in mutant cells (Fig. 4A, panel g) did not differ from that in wild-type cells (Fig. 4A, panel e). In both cell types, Igl was localized to a significant number of cytoplasmic puncta. Together, these data suggest that EhRabA may directly or indirectly regulate the trafficking of a subset of vesicles that are also responsible for the localization of Hgl and Lgl, but not of Igl. The data also demonstrate that Igl may be transported by vesicle trafficking routes that differ from that of the Hgl-Lgl heterodimer.
Western blot analysis demonstrated that the levels of at least two Gal/GalNAc subunits, Hgl and Igl, were not altered in the mutant compared to wild-type parental cells (Fig. 4B). Therefore, the differences observed between the mutant and wild-type cell lines were not likely the result of changes in protein levels. Given that antibodies recognize multiple isoforms of Lgl in trophozoites (25), the level of this subunit was not assessed by Western blot analysis.
Hgl-positive compartments colocalize with markers of the ER but not of the Golgi apparatus.
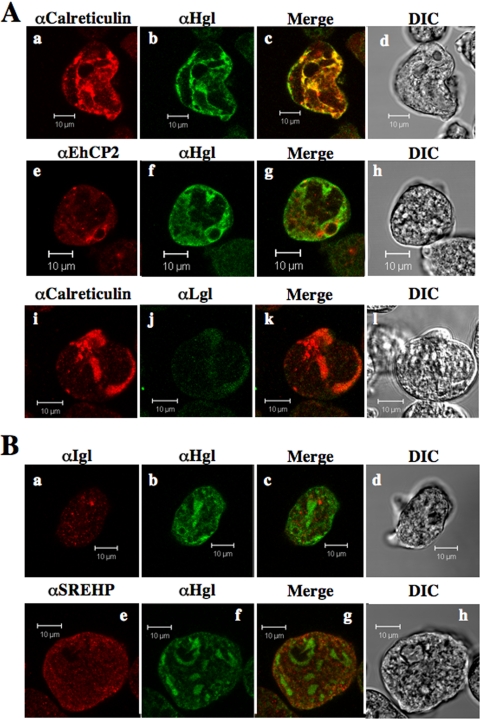
The novel Hgl-containing organelle observed in the mutants was reminiscent of recently described tubular compartments of the ER of E. histolytica (41). Therefore, we counterstained these organelles with an antibody specific for E. histolytica calreticulin (9), an ER-resident protein. Since the ER often assumes a perinuclear organization, we simultaneously stained cells with propidium iodide, a nuclear stain. Minimal colocalization of Hgl (Fig. 5A panels a and b) or Lgl (Fig. 5B, panels a and b) and calreticulin was observed in wild-type cells, whereas significant colocalization of the these antigens was observed in mutants (Fig. 5A, panels e and f and B, panels e and f). Significant colocalization of Hgl and Lgl with calreticulin suggests that both subunits are mislocalized to the ER or the same novel compartment derived from the ER. We also observed that the novel organelle was perinuclear in its localization (Fig. 5A, panel g), further supporting the ER origin of this organelle.
FIG. 5.
The heavy (A) and light (B) subunits of the Gal/GalNac lectin colocalize with calreticulin (ER marker). IF microscopy results are shown for wild-type (A, panels a to c, and B, panels a to c) and mutant cells (A, panels e to g, and B, panels e to g) using anti-Hgl (A) or anti-Lgl (B) and anti-calreticulin antibodies. The tubular compartment is perinuclear as determined by propidium iodide staining (A, panel g). Colocalized antigens are shown in light blue (A, panels c and g) or yellow (B, panels c and g), and corresponding merged differential interference contrast microscopic images (A, panels d and h, and B, panels d and h) are provided. Bars, 10 μm.
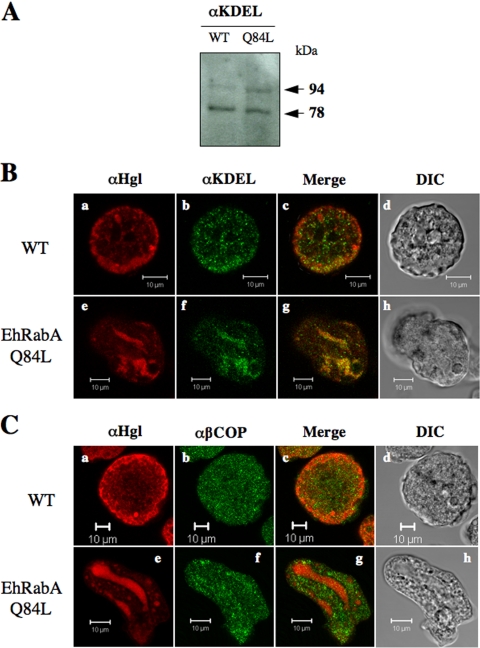
To confirm the ER origin of the novel compartment we also used an antibody specific for the ER retention signal, KDEL. This antibody recognizes an array of proteins on Western blots of mammalian cell lysates; however, it prominently stains two mammalian ER-resident proteins, GRP78 (78 kDa) and GRP94 (94 kDa) (27). Likewise, Western blot analysis of trophozoite lysates from untransformed and mutant E. histolytica revealed an array of proteins (data not shown) with two prominent bands of 78 and 94 kDa (Fig. 6A). This suggested that this antibody might be used to authentically label the ER in E. histolytica trophozoites. While the Western blot assay was performed only to assess the utility of this antibody, it revealed that the 94-kDa species was more abundant in the mutant than in the wild-type cell line. This may indicate an expansion of the ER as a result of EhRabAQ84L expression. IF microscopy with the anti-KDEL antibody revealed minimal colocalization of Hgl with the KDEL marker in wild-type cells (Fig. 6B, panels a to c) but near-complete colocalization of Hgl with the ER marker in the mutant (Fig. 6B, panels e to g). This provides support for the ER origin of this organelle.
FIG. 6.
The heavy subunit of the Gal/GalNac lectin colocalizes with KDEL (ER marker), but not β-COP (Golgi marker), in cells expressing EhRabAQ84L. (A) Western blot analysis of trophozoite lysates from wild-type (WT) and mutant (Q84L) cells using anti-KDEL antibody. Consistent with its specificity in mammalian cells, the antibody prominently recognizes two proteins of 78 kDa and 94 kDa. (B) IF microscopy of wild-type (a to c) and mutant (e to g) cells by using anti-Hgl or anti-KDEL antibodies. (C) IF microscopy of wild-type (a to c) and mutant (e to g) cells using anti-Hgl or anti-β-COP antibodies. In both panels B and C, colocalized antigens are shown in yellow (c and g) and corresponding differential interference contrast microscopic images (d and h) are provided. Bars, 10 μm.
To determine if the novel compartments arose by fusion events between the ER and Golgi complex, we counterstained wild-type and mutant cells with an antibody specific for β-COP. This antibody was previously shown to label Golgi complex-like elements in this organism (46). We observed minimal colocalization of Hgl and the Golgi antigen in the mutant cell line, suggesting that little mixing of these organelles occurred as a result of EhRabAQ84L expression (Fig. 6C).
Expression of GTP-bound EhRabA alters the subcellular localization of a secreted cysteine protease, EhCP2.
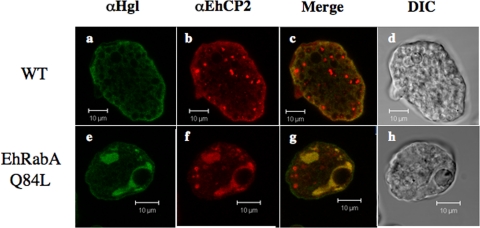
We have shown that expression of EhRabAQ84L results in aberrant trafficking of at least one transmembrane protein, Hgl. To test the effect of mutant EhRabA expression on another class of secretory proteins, namely, soluble proteins, we looked at subcellular localization of a secreted cysteine protease, EhCP2, by IF microscopy. Like Hgl and Lgl, EhCP2 was mislocalized to large tubular compartments in the mutant cell line (Fig. 7). This suggests that the molecular machinery governing the trafficking of Hgl and Lgl may be shared by this cysteine protease and that EhRabA may directly or indirectly regulate this pathway.
FIG. 7.
Subcellular localization of the Hgl and EhCP2 subunits in wild-type cells and cells expressing EhRabAQ84L. IF microscopy of wild-type (a to c) and mutant (e to g) cells by using anti-Hgl or anti-EhCP2 antibodies. Colocalized antigens are shown in yellow (c and g), and corresponding differential interference contrast (DIC) (d and h) microscopic images are provided. In wild-type cells, Hgl and EhCP2 are localized to the plasma membrane and cytoplasmic puncta. Mutant cells exhibit near-complete colocalization in intracellular compartments, some of which are perinuclear. Bars, 10 μm.
Expression of GTP-bound EhRabA does not alter the localization of SREHP, a serine-rich protein of E. histolytica.
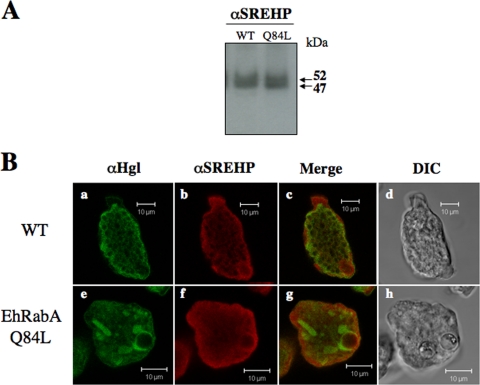
It has been proposed that a putatively lipid-anchored cell surface serine-rich E. histolytica protein, SREHP, also participates in the uptake of hRBCs by phagocytosis (42). Therefore, we examined the cellular levels and localization of SREHP in mutant and wild-type cells. Western blot (Fig. 8A) and IF (Fig. 8B) analyses indicated that both the levels and localization of SREHP remained unchanged in the mutants. In both wild-type and mutant cells SREHP localized to the plasma membrane and to an intracellular network (Fig. 8B). Furthermore, like Igl, SREHP did not colocalize with Hgl in the novel compartment (Fig. 8B).
FIG. 8.
Subcellular localization of SREHP is unchanged in cells expressing EhRabAQ84L. (A) Western blot analysis of trophozoite lysates from wild-type (WT) and mutant (AQ84L) cells using anti-SREHP antibody. Consistent with a previous report (50), the antibody recognizes a series of proteins ranging in size from 47 to 52 kDa. There were no apparent changes in the level of SREHP in the mutant cell line. (B) IF microscopy of wild-type (a to c) and mutant (e to g) cells using anti-Hgl (a and e) or anti-SREHP (b and f) antibodies. Colocalized antigens are shown in yellow (c and g), and corresponding differential interference contrast microscopic images (d and h) are provided. In wild-type cells, both Hgl and SREHP are enriched at the plasma membrane and localize to intracellular puncta. In mutant cells, SREHP appears to retain its plasma membrane enrichment, whereas Hgl localizes to intracellular and, in some cases, perinuclear compartments. Bars, 10 μm.
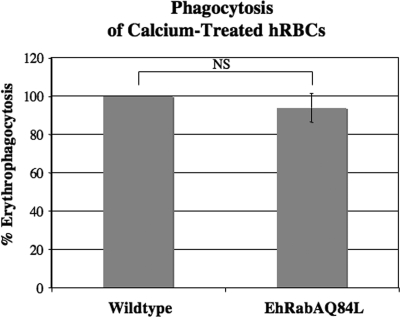
It has been reported that E. histolytica trophozoites preferentially phagocytose apoptotic host cells (10) and that SREHP may serve as a receptor for such targets (42). Since the localization of SREHP was unchanged in the EhRabAQ84L-expressing cells, we predicted that these mutants would take up apoptotic targets as efficiently as untransformed cells. Therefore, we tested the ability of the mutants to internalize erythrocytes that were pretreated with calcium. Such treatment induces the exposure of phosphatidylserine on the outer membrane of the hRBCs, which is a hallmark of apoptosis (3) As predicted, phagocytosis of calcium-treated cells was not inhibited by expression of EhRabAQ84L (Fig. 9), suggesting that the mutant cells could efficiently adhere to apoptotic cells. Fixation of mammalian cells with paraformaldehyde can also induce exposure of phosphatidylserine on outer membrane leaflets (49), recapitulating the apoptotic phenotype. Therefore, we also tested the ability of mutants and wild-type cells to adhere to fixed epithelial monolayers. Adhesion of mutant cells to paraformaldehyde-fixed monolayers of CHO cells was comparable to that of wild-type parental cells (data not shown), further supporting the idea that expression of EhRabAQ84L does not inhibit trafficking of cell surface receptors specific for apoptotic host cells.
FIG. 9.
Interactions with senescing host cells are unaltered in the mutant cell line. Phagocytosis of calcium-treated erythrocytes was not altered by expression of EhRabAQ84L. Data represent the means ± SD of four trials and are reported as the percentage of uptake by wild-type cells, which was arbitrarily set to 100%. (NS, nonsignificant.)
Treatment with BFA phenocopies expression of EhRabAQ84L.
BFA is a fungal metabolite that can disrupt secretory pathway vesicle trafficking (11). For example, in mammalian cells, this reagent can block egress of vesicles from the ER (21) and can induce vesicularization of Golgi apparata (11). In E. histolytica, BFA can alter the morphology of a population of Golgi-specific vesicles and inhibit the trafficking of a subset of secretory proteins (15). Interestingly, in E. histolytica, BFA also induces the formation of tubular structures that colocalize with an ER marker, protein disulfide isomerase (15). These structures are similar to those observed in the EhRabAQ84L-expressing cell line; therefore, we explored whether EhRabA might regulate vesicle trafficking pathways that are also sensitive to BFA treatment. We examined the localization of several cell surface and secreted proteins after BFA treatment of wild-type cells. Interestingly, exposure to BFA recapitulated the EhRabAQ84L-induced phenotype in that Hgl, Lgl, and EhCP2 were aberrantly localized to a large intracellular tubular network (Fig. 10A), whereas the distributions of Igl and SREHP were unchanged (Fig. 10B). This suggests that EhRabA participates in vesicle trafficking pathways that are also sensitive to BFA.
FIG. 10.
Subcellular localization of secretory proteins after treatment of wild-type cells with brefeldin A. Cells were treated with 100 μg/ml BFA for 3 h, after which they were fixed and stained with antibodies specific for a variety of secretory proteins. (A) BFA treatment phenocopies expression of EhRabAQ84L with respect to the localization of Hgl (b and f), calreticulin (a and i), Lgl (j), and EhCP2 (e). In treated cells these proteins exhibit near-complete colocalization in intracellular compartments, some of which are perinuclear. (B) Similar to EhRabAQ84L overexpression, BFA does not alter the localization of Igl or SREHP. Colocalized antigens are shown in yellow (A, panels c, g, and k, and B, panels c and g) and corresponding differential interference contrast images (A, panels d, h, and l, and B, panels d and h) are provided. Bars, 10 μm.
DISCUSSION
We have demonstrated that overexpression of a GTP-bound mutant of EhRabA results in decreased phagocytosis of nonsenescing red blood cells but not of senescing red blood cells. Overexpression of the mutant protein also induced the appearance of large tubular and perinuclear organelles that could be stained with ER-specific marker antibodies, suggesting that this novel compartment was of ER origin. The appearance of the tubular organelles in the mutant cells supports the recent finding that E. histolytica trophozoites possess a traditional continuous ER (41).
The nature of the phagocytic defect in the mutants was likely related to their inability to adhere efficiently to the targets. This may have been caused by mislocalization of two subunits of the Gal/GalNAc lectin, Hgl and Lgl, to the novel ER-like compartment. In these mutants, at least one other secretory protein, EhCP2, was also mislocalized to the ER-like organelles; however, the cellular localization of two other cell surface proteins, Igl and SREHP, was unchanged. Interestingly, the EhRabAQ84L phenotype was similar to that induced by treatment with BFA. Together, the data suggest that EhRabA directly or indirectly influences the morphological integrity of the ER and the trafficking of a subset of secretory proteins in E. histolytica. This report is the first to demonstrate that distinct secretory pathways may exist in E. histolytica.
Point mutations in the GTPase domain of Rabs, which result in reduced GTPase activity, can lead to either gain-of-function (18) or loss-of-function phenotypes (35). Constitutively GTP-bound Rabs are generally considered to be hyperactivated and, therefore, have acquired a gain-of-function phenotype. However, in cases where a terminal vesicle fusion event relies on GTP hydrolysis, overexpression of a hydrolase-dead mutant may, in fact, result in a loss-of-function phenotype. It is not known if the engineered glutamine-to-leucine point mutation described here results in gain of function or loss of function for EhRabA.
The localization of EhRabA to membrane protrusions (48) suggests a role in late secretion. If overexpression of the hydrolyase-dead version of EhRabA blocks this process (loss of function), it may lead to accumulation of cargo in secretory organelles or an imbalance between anterograde and retrograde trafficking in favor of retrograde movement of secretory proteins toward the ER. Localization of EhRabA near the plasma membrane (48) may also suggest a role in regulating retrograde vesicle trafficking. Expression of the GTP-bound form of this Rab might accelerate retrograde vesicle movement (gain of function) toward the ER. In all of these cases, secretory proteins would accumulate in the ER, which may lead to an expansion of the ER (32).
It is not surprising that alterations in Rab function can lead to changes in organellar morphology. Rabs influence the morphology of organelles by regulating intraorganellar or interorganellar fusion events and/or by regulating the entry in or exit of transport vesicles. Since EhRabA does not localize to the ER (48) it is possible that it is acting in trans through effector proteins to promote intraorganellar or interorganellar fusion of ER-derived compartments. In support of this, overexpression of GTP-bound Rab5 in Caenorhabditis elegans results in the appearance of large fused ER-derived compartments reminiscent of those observed in this study (1). Like EhRabA, C. elegans Rab5 does not localize to the ER but is thought to promote intraorganellar fusion of the ER through effector proteins.
A striking result of this study was that BFA treatment could recapitulate the novel organellar phenotype associated with overexpression of EhRabAQ84L. BFA is known to block ER-to-Golgi trafficking (21), which could alter the balance between anterograde and retrograde vesicle traffic. The similar phenotype in the EhRabAQ84L-expressing cells may support a model in which trafficking to and from the ER is perturbed. However, there is not complete phenotypic recapitulation by BFA treatment, since others have shown that BFA-treated E. histolytica cells do not exhibit reduced phagocytosis of at least one phagocytic target, green fluorescent protein-expressing E. coli (15). Therefore, it is likely that the resultant phenotype of the mutant occurred through a different process than that operating in BFA-treated cells. Importantly, BFA induces alterations in the secretory pathway by impairing the association and dissociation of coat proteins on cellular membranes (11), whereas Rabs are not thought to participate in the assembly or disassembly of such protein coats.
It is interesting that the localizations of Igl and SREHP were unaffected by EhRabAQ84L expression. While Igl is proposed to interact with the membrane via a GPI-anchored protein, the lipid modification connecting SREHP to the membrane is not known. GPI-anchored proteins can exit the ER in distinct vesicles from other secretory proteins (20). Our data suggest that such GPI-specific secretory pathways similarly exist in E. histolytica. Although Lgl has also been proposed to be GPI anchored (14), its location must be significantly influenced by its covalent attachment to Hgl. It was also intriguing that the intermediate subunit of the Gal/GalNAc lectin was properly localized even when the heavy and light chains exhibited aberrant localization. This suggests that temporal and spatial regulation of Igl may differ from that of the Hgl-Lgl heterodimer, which may have implications in the control of lectin function, and thus virulence. This mutant provides a model cell line in which such questions might be explored. Since at least two cell surface proteins, Igl and SREHP, did not exhibit aberrant localization, we do not believe that all secretory trafficking was perturbed in the mutant or that the cells were under general metabolic stress, which might lead to organelle expansion and dysfunction.
Expression of both the GDP-bound (47) and GTP-bound (this study) forms of EhRabA leads to mislocalization Hgl. It remains to be determined if aberrant localization of the Hgl and other secretory proteins in mutant cell lines is a direct effect of the perturbation of EhRabA function or an indirect effect of organellar reorganization. Given the importance of the Gal/GalNAc lectin to pathogenicity, distinguishing between these two possibilities will provide significant insight into virulence mechanisms of E. histolytica.
Acknowledgments
We thank W. A. Petri, Jr. (University of Virginia, Charlottesville), N. Guillén (Unité de Biologie Cellulaire du Parasitisme, Institut Pasteur, Paris, France), and S. L. Stanley, Jr. (Washington University School of Medicine, St. Louis, MO) for antibody reagents. We thank the members of the Temesvari laboratory for critical reading of the manuscript and helpful discussions.
The project described was supported by grant no. R01AI046414 from the National Institute of Allergy and Infectious Diseases. This material is based upon work supported by CSREES/USDA, under project number SC-1700312 (Technical Contribution No. 5570 of the Clemson University Experiment Station).
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Allergy and Infectious Diseases, the National Institutes of Health, or the U.S. Department of Agriculture.
Footnotes
Published ahead of print on 17 April 2009.
REFERENCES
- 1.Audhya, A., A. Desai, and K. Oegema. 2007. A role for Rab5 in structuring the endoplasmic reticulum. J. Cell Biol. 17843-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boettner, D. R., C. D. Huston, A. S. Linford, S. N. Buss, E. Houpt, N. E. Sherman, and W. A. Petri, Jr. 2008. Entamoeba histolytica phagocytosis of human erythrocytes involves PATMK, a member of the transmembrane kinase family. PLoS Pathog. 4e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boettner, D. R., C. D. Huston, J. A. Sullivan, and W. A. Petri, Jr. 2005. Entamoeba histolytica and Entamoeba dispar utilize externalized phosphatidylserine for recognition and phagocytosis of erythrocytes. Infect. Immun. 733422-3430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Buczynski, G., J. Bush, L. Zhang, J. Rodriguez-Paris, and J. Cardelli. 1997. Evidence for a recycling role for Rab7 in regulating a late step in endocytosis and in retention of lysosomal enzymes in Dictyostelium discoideum. Mol. Biol. Cell 81343-1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bush, J., L. Temesvari, J. Rodriguez-Paris, G. Buczynski, and J. Cardelli. 1996. A role for a Rab4-like GTPase in endocytosis and in regulation of contractile vacuole structure and function in Dictyostelium discoideum. Mol. Biol. Cell 71623-1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chadee, K., W. A. Petri, Jr., D. J. Innes, and J. I. Ravdin. 1987. Rat and human colonic mucins bind to and inhibit adherence lectin of Entamoeba histolytica. J. Clin. Investig. 801245-1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Diamond, L. S., D. R. Harlow, and C. C. Cunnick. 1978. A new medium for the axenic cultivation of Entamoeba histolytica and other Entamoeba. Trans. R. Soc. Trop. Med. Hyg. 72431-432. [DOI] [PubMed] [Google Scholar]
- 8.Ghosh, S. K., J. Field, M. Frisardi, B. Rosenthal, Z. Mai, R. Rogers, and J. Samuelson. 1999. Chitinase secretion by encysting Entamoeba invadens and transfected Entamoeba histolytica trophozoites: localization of secretory vesicles, endoplasmic reticulum, and Golgi apparatus. Infect. Immun. 673073-3081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Girard-Misguich, F., M. Sachse, J. Santi-Rocca, and N. Guillén. 2008. The endoplasmic reticulum chaperone calreticulin is recruited to the uropod during capping of surface receptors in Entamoeba histolytica. Mol. Biochem. Parasitol. 157236-240. [DOI] [PubMed] [Google Scholar]
- 10.Huston, C. D., D. R. Boettner, V. Miller-Sims, and W. A. Petri, Jr. 2003. Apoptotic killing and phagocytosis of host cells by the parasite Entamoeba histolytica. Infect. Immun. 71964-972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Klausner, R. D., J. G. Donaldson, and J. Lippincott-Schwartz. 1992. Brefeldin A: insights into the control of membrane traffic and organelle structure. J. Cell Biol. 1161071-1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Laughlin, R. C., G. C. McGugan, R. R. Powell, B. H. Welter, and L. A. Temesvari. 2004. Involvement of raft-like plasma membrane domains of Entamoeba histolytica in pinocytosis and adhesion. Infect. Immun. 725349-5357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Leung, K. F., R. Baron, B. R. Ali, A. I. Magee, and M. C. Seabra. 2007. Rab GTPases containing a CAAX motif are processed post-geranylgeranylation by proteolysis and methylation. J. Biol. Chem. 2821487-1497. [DOI] [PubMed] [Google Scholar]
- 14.Mann, B. J. 2002. Structure and function of the Entamoeba histolytica Gal/GalNAc lectin. Int. Rev. Cytol. 21659-80. [DOI] [PubMed] [Google Scholar]
- 15.Manning-Cela, R., C. Marquez, E. Franco, P. Talamas-Rohana, and I. Meza. 2003. BFA-sensitive and insensitive exocytic pathways in Entamoeba histolytica trophozoites: their relationship to pathogenesis. Cell. Microbiol. 5921-932. [DOI] [PubMed] [Google Scholar]
- 16.Marion, S., and N. Guillén. 2006. Genomic and proteomic approaches highlight phagocytosis of living and apoptotic human cells by the parasite Entamoeba histolytica. Int. J. Parasitol. 36131-139. [DOI] [PubMed] [Google Scholar]
- 17.Marion, S., C. Laurent, and N. Guillén. 2005. Signalization and cytoskeleton activity through myosin IB during the early steps of phagocytosis in Entamoeba histolytica: a proteomic approach. Cell. Microbiol. 71504-1518. [DOI] [PubMed] [Google Scholar]
- 18.Martinez, O., C. Antony, G. Pehau-Arnaudet, E. G. Berger, J. Salamero, and B. Goud. 1997. GTP-bound forms of rab6 induce the redistribution of Golgi proteins into the endoplasmic reticulum. Proc. Natl. Acad. Sci. USA 941828-1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mazzuco, A., M. Benchimol, and W. De Souza. 1997. Endoplasmic reticulum and Golgi-like elements in Entamoeba. Micron 28241-247. [DOI] [PubMed] [Google Scholar]
- 20.Morsomme, P., and H. Riezman. 2002. The Rab GTPase Ypt1p and tethering factors couple protein sorting at the ER to vesicle targeting to the Golgi apparatus. Dev. Cell 2307-317. [DOI] [PubMed] [Google Scholar]
- 21.Nuchtern, J. G., J. S. Bonifacino, W. E. Biddison, and R. D. Klausner. 1989. Brefeldin A implicates egress from endoplasmic reticulum in class I restricted antigen presentation. Nature 339223-226. [DOI] [PubMed] [Google Scholar]
- 22.Okada, M., C. D. Huston, B. J. Mann, W. A. Petri, Jr., K. Kita, and T. Nozaki. 2005. Proteomic analysis of phagocytosis in the enteric protozoan parasite Entamoeba histolytica. Eukaryot. Cell 4827-831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Okada, M., and T. Nozaki. 2006. New insights into molecular mechanisms of phagocytosis in Entamoeba histolytica by proteomic analysis. Arch. Med. Res. 37244-252. [DOI] [PubMed] [Google Scholar]
- 24.Olivos-Garcia, A., E. Tello, M. Nequiz-Avendano, A. Gonzalez-Canto, R. Lopez-Vancell, M. C. Garcia de Leon, I. Montfort, and R. Perez-Tamayo. 2004. Cysteine proteinase activity is required for survival of the parasite in experimental acute amoebic liver abscesses in hamsters. Parasitology 12919-25. [DOI] [PubMed] [Google Scholar]
- 25.Petri, W. A., Jr., M. D. Chapman, T. Snodgrass, B. J. Mann, J. Broman, and J. I. Ravdin. 1989. Subunit structure of the galactose and N-acetyl-D-galactosamine-inhibitable adherence lectin of Entamoeba histolytica. J. Biol. Chem. 2643007-3012. [PubMed] [Google Scholar]
- 26.Pimenta, P. F., L. S. Diamond, and D. Mirelman. 2002. Entamoeba histolytica Schaudinn, 1903 and Entamoeba dispar Brumpt, 1925: differences in their cell surfaces and in the bacteria-containing vacuoles. J. Eukaryot. Microbiol. 49209-219. [DOI] [PubMed] [Google Scholar]
- 27.Pinto, M. P., C. P. Grou, I. S. Alencastre, M. E. Oliveira, C. Sa-Miranda, M. Fransen, and J. E. Azevedo. 2006. The import competence of a peroxisomal membrane protein is determined by Pex19p before the docking step. J. Biol. Chem. 28134492-34502. [DOI] [PubMed] [Google Scholar]
- 28.Powell, R. R., and L. A. Temesvari. 2004. Involvement of a Rab8-like protein of Dictyostelium discoideum, Sas1, in the formation of membrane extensions, secretion and adhesion during development. Microbiology 1502513-2525. [DOI] [PubMed] [Google Scholar]
- 29.Powell, R. R., B. H. Welter, R. Hwu, B. Bowersox, C. Attaway, and L. A. Temesvari. 2006. Entamoeba histolytica: FYVE-finger domains, phosphatidylinositol 3-phosphate biosensors, associate with phagosomes but not fluid filled endosomes. Exp. Parasitol. 112221-231. [DOI] [PubMed] [Google Scholar]
- 30.Ramakrishnan, G., R. R. Vines, B. J. Mann, and W. A. Petri Jr. 1997. A tetracycline-inducible gene expression system in Entamoeba histolytica. Mol. Biochem. Parasitol. 8493-100. [DOI] [PubMed] [Google Scholar]
- 31.Ravdin, J. I., and R. L. Guerrant. 1981. Role of adherence in cytopathogenic mechanisms of Entamoeba histolytica. Study with mammalian tissue culture cells and human erythrocytes. J. Clin. Investig. 681305-1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rivera, V. M., X. Wang, S. Wardwell, N. L. Courage, A. Volchuk, T. Keenan, D. A. Holt, M. Gilman, L. Orci, F. Cerasoli, Jr., J. E. Rothman, and T. Clackson. 2000. Regulation of protein secretion through controlled aggregation in the endoplasmic reticulum. Science 287826-830. [DOI] [PubMed] [Google Scholar]
- 33.Rodriguez, M. A., and E. Orozco. 1986. Isolation and characterization of phagocytosis- and virulence-deficient mutants of Entamoeba histolytica. J. Infect. Dis. 15427-32. [DOI] [PubMed] [Google Scholar]
- 34.Saito-Nakano, Y., B. J. Loftus, N. Hall, and T. Nozaki. 2005. The diversity of Rab GTPases in Entamoeba histolytica. Exp. Parasitol. 110244-252. [DOI] [PubMed] [Google Scholar]
- 35.Saito-Nakano, Y., T. Yasuda, K. Nakada-Tsukui, M. Leippe, and T. Nozaki. 2004. Rab5-associated vacuoles play a unique role in phagocytosis of the enteric protozoan parasite Entamoeba histolytica. J. Biol. Chem. 27949497-49507. [DOI] [PubMed] [Google Scholar]
- 36.Schafer, W. R., and J. Rine. 1992. Protein prenylation: genes, enzymes, targets, and functions. Annu. Rev. Genet. 26209-237. [DOI] [PubMed] [Google Scholar]
- 37.Smeland, T. E., M. C. Seabra, J. L. Goldstein, and M. S. Brown. 1994. Geranylgeranylated Rab proteins terminating in Cys-Ala-Cys, but not Cys-Cys, are carboxyl-methylated by bovine brain membranes in vitro. Proc. Natl. Acad. Sci. USA 9110712-10716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stanley, S. L., Jr., T. Zhang, D. Rubin, and E. Li. 1995. Role of the Entamoeba histolytica cysteine proteinase in amebic liver abscess formation in severe combined immunodeficient mice. Infect. Immun. 631587-1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stenmark, H., and V. M. Olkkonen. 2001. The Rab GTPase family. Genome Biol. 2:REVIEWS3007. [DOI] [PMC free article] [PubMed]
- 40.Stenmark, H., R. G. Parton, O. Steele-Mortimer, A. Lutcke, J. Gruenberg, and M. Zerial. 1994. Inhibition of rab5 GTPase activity stimulates membrane fusion in endocytosis. EMBO J. 131287-1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Teixeira, J. E., and C. D. Huston. 2008. Evidence of a continuous endoplasmic reticulum in the protozoan parasite Entamoeba histolytica. Eukaryot. Cell 71222-1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Teixeira, J. E., and C. D. Huston. 2008. Participation of the serine-rich Entamoeba histolytica protein in amebic phagocytosis of apoptotic host cells. Infect. Immun. 76959-966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vines, R. R., J. E. Purdy, B. D. Ragland, J. Samuelson, B. J. Mann, and W. A. Petri, Jr. 1995. Stable episomal transfection of Entamoeba histolytica. Mol. Biochem. Parasitol. 71265-267. [DOI] [PubMed] [Google Scholar]
- 44.Vitale, A., and J. Denecke. 1999. The endoplasmic reticulum-gateway of the secretory pathway. Plant Cell 11615-628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Voigt, H., J. C. Olivo, P. Sansonetti, and N. Guillén. 1999. Myosin IB from Entamoeba histolytica is involved in phagocytosis of human erythrocytes. J. Cell Sci. 1121191-1201. [DOI] [PubMed] [Google Scholar]
- 46.Welter, B. H., R. C. Laughlin, and L. A. Temesvari. 2002. Characterization of a Rab7-like GTPase, EhRab7: a marker for the early stages of endocytosis in Entamoeba histolytica. Mol. Biochem. Parasitol. 121254-264. [DOI] [PubMed] [Google Scholar]
- 47.Welter, B. H., R. R. Powell, M. Leo, C. M. Smith, and L. A. Temesvari. 2005. A unique Rab GTPase, EhRabA, is involved in motility and polarization of Entamoeba histolytica cells. Mol. Biochem. Parasitol. 140161-173. [DOI] [PubMed] [Google Scholar]
- 48.Welter, B. H., and L. A. Temesvari. 2004. A unique Rab GTPase, EhRabA, of Entamoeba histolytica, localizes to the leading edge of motile cells. Mol. Biochem. Parasitol. 135185-195. [DOI] [PubMed] [Google Scholar]
- 49.Wong, K., X. Li, and Y. Ma. 2006. Paraformaldehyde induces elevation of intracellular calcium and phosphatidylserine externalization in platelets. Thromb. Res. 117537-542. [DOI] [PubMed] [Google Scholar]
- 50.Zhang, T., P. R. Cieslak, L. Foster, C. Kunz-Jenkins, and S. L. Stanley Jr. 1994. Antibodies to the serine rich Entamoeba histolytica protein (SREHP) prevent amoebic liver abscess in severe combined immunodeficient (SCID) mice. Parasite Immunol. 16225-230. [DOI] [PubMed] [Google Scholar]