Abstract
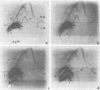
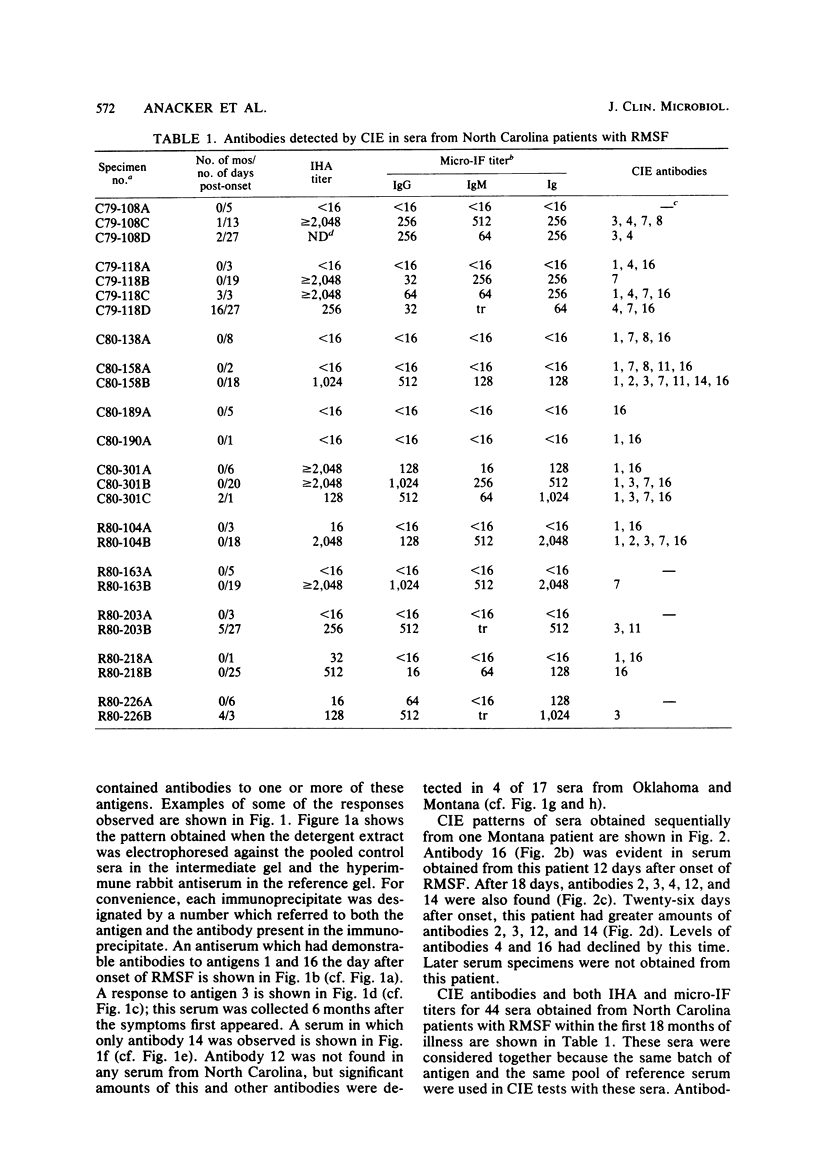
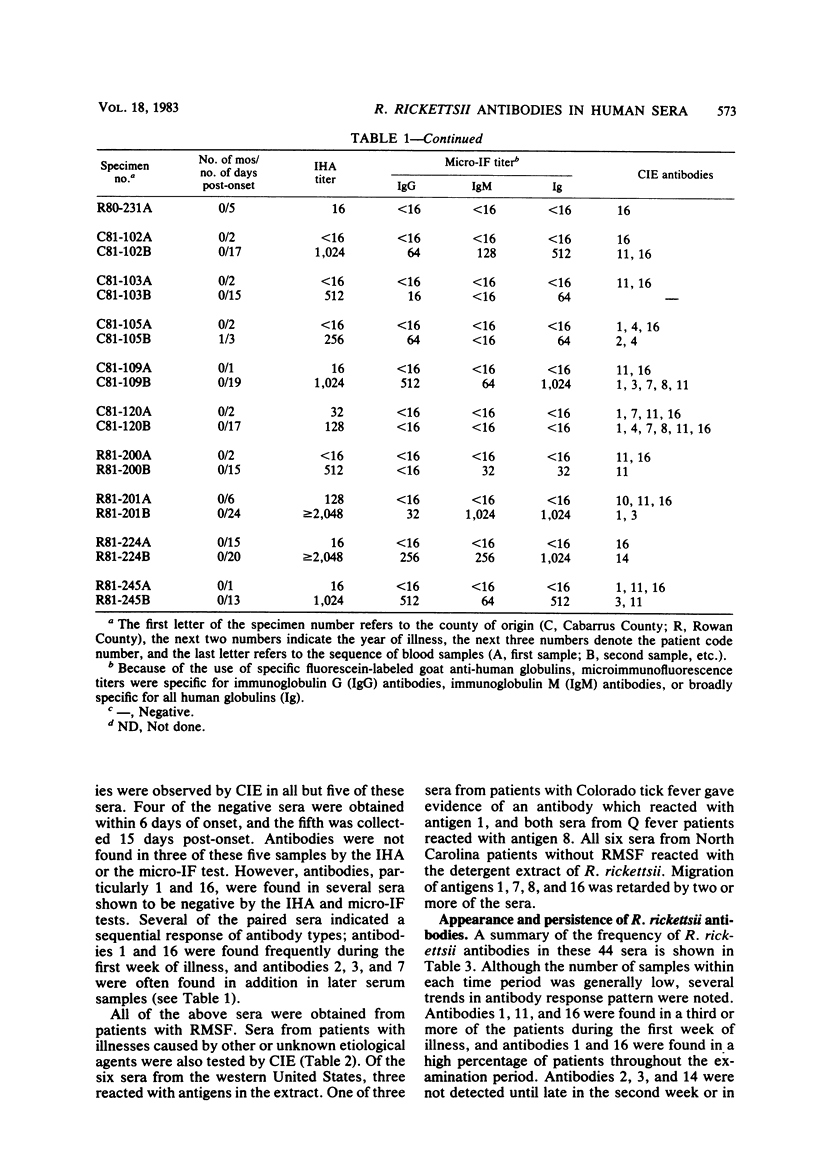
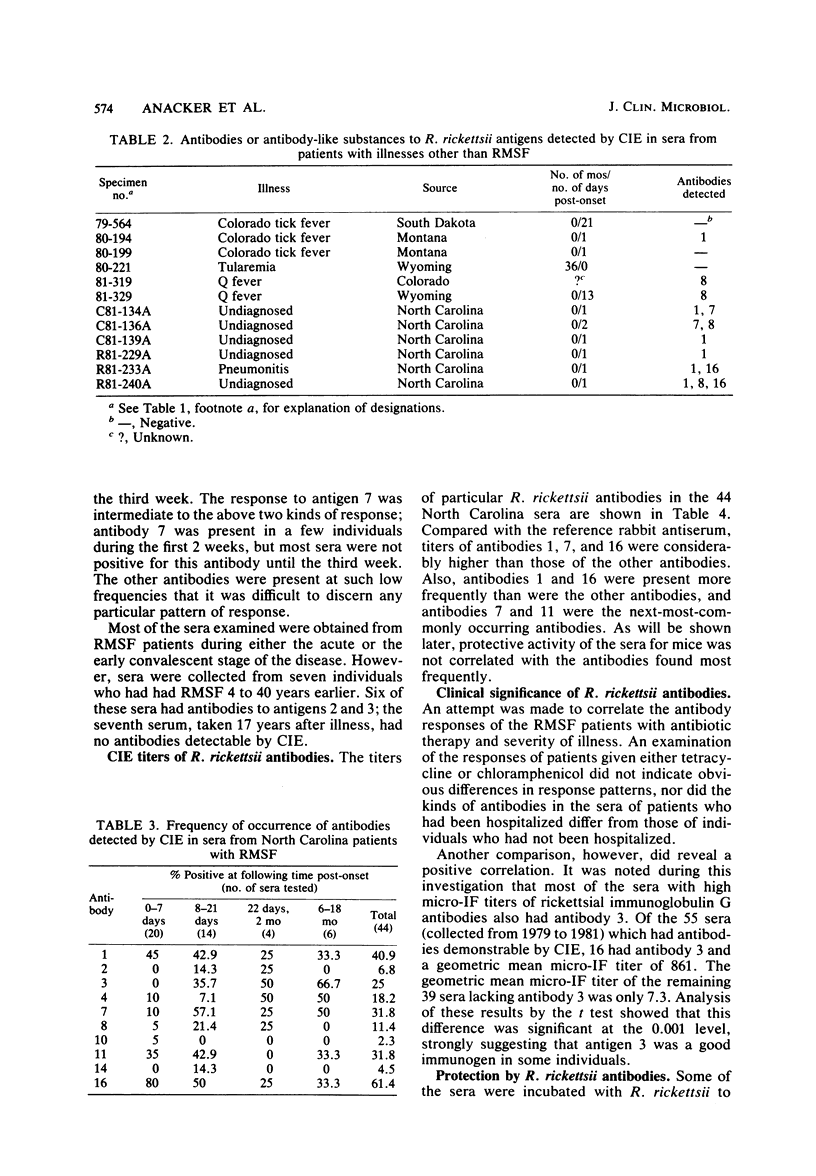
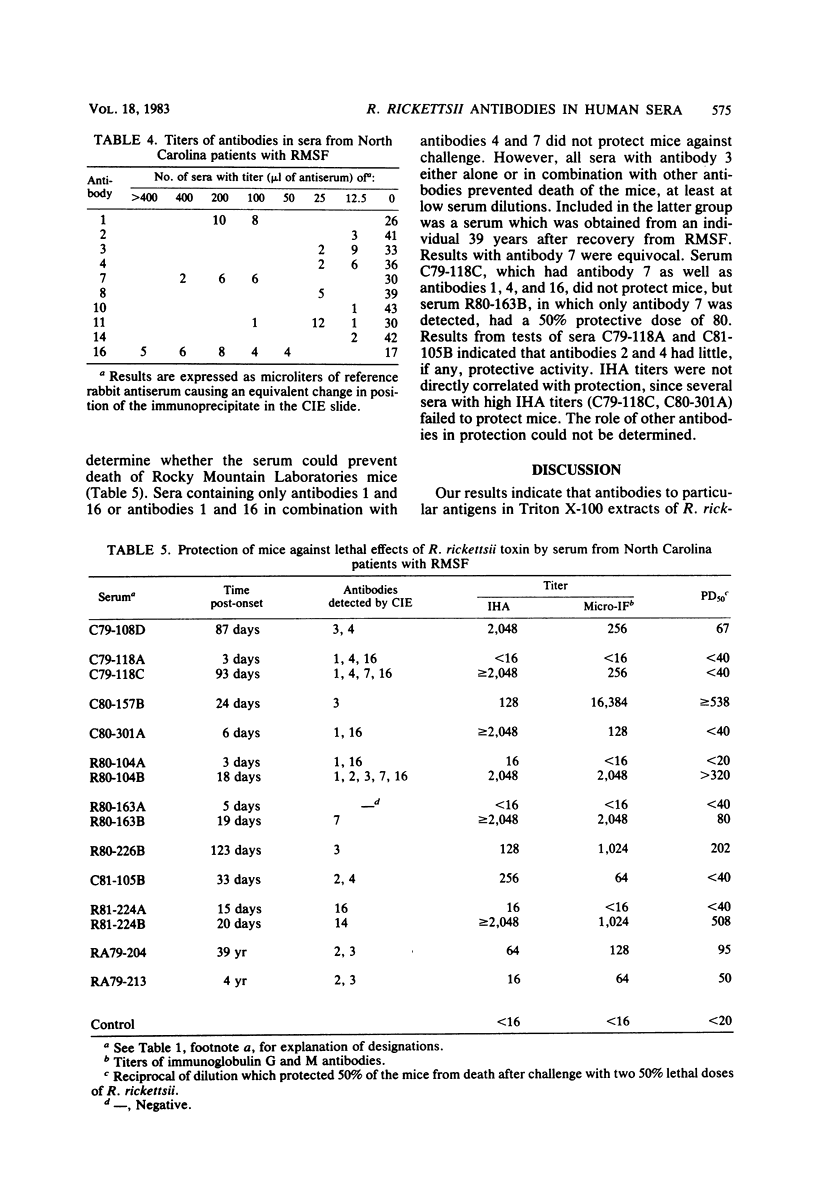
To identify Rickettsia rickettsii antigens of immunological importance, we examined sera from patients with serologically confirmed cases of Rocky Mountain spotted fever by crossed immunoelectrophoresis for antibodies to antigens extracted from the R strain of R. rickettsii with the detergent Triton X-100. Sixteen antigens were identified in the detergent extract by crossed immunoelectrophoresis with a hyperimmune rabbit serum raised against whole rickettsiae. When the rabbit antiserum was placed in the reference gel and patient sera were placed in the intermediate gel, antibodies to one or more antigens were detected in 61 of 71 North Carolina sera, all of 7 Oklahoma sera, and 9 of 10 Montana sera obtained from 1 day to 40 years after onset of Rocky Mountain spotted fever. Antibodies to antigens 1 and 16 were found as early as 1 day after onset of illness, and antibody to 16 was found in 20 of 29 sera obtained within the first 7 days of illness. Antibodies to antigens 2 and 3 generally did not appear until the third week of illness but were found in six of seven serum samples collected 4 to 40 years after onset of Rocky Mountain spotted fever. Antibodies to R. rickettsii antigens 1, 7, 8, and 16 were found in sera from patients with illnesses caused by other etiological agents. Four of the Oklahoma and Montana sera from Rocky Mountain spotted fever patients, but none of the North Carolina sera, had antibodies to antigen 12. Sera containing antibodies against antigens 3 and 14 prevented death of mice challenged with two 50% lethal doses of R. rickettsii.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anacker R. L., Gerloff R. K., Thomas L. A., Mann R. E., Brown W. R., Bickel W. D. Purification of Rickettsia rickettsi by density-gradient zonal centrifugation. Can J Microbiol. 1974 Nov;20(11):1523–1527. doi: 10.1139/m74-238. [DOI] [PubMed] [Google Scholar]
- Anacker R. L., McCaul T. F., Burgdorfer W., Gerloff R. K. Properties of selected rickettsiae of the spotted fever group. Infect Immun. 1980 Feb;27(2):468–474. doi: 10.1128/iai.27.2.468-474.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anacker R. L., Philip R. N., Thomas L. A., Casper E. A. Indirect hemagglutination test for detection of antibody to Rickettsia rickettsii in sera from humans and common laboratory animals. J Clin Microbiol. 1979 Nov;10(5):677–684. doi: 10.1128/jcm.10.5.677-684.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BELL E. J., PICKENS E. G. A toxic substance associated with the rickettsias of the spotted fever group. J Immunol. 1953 May;70(5):461–472. [PubMed] [Google Scholar]
- BELL E. J., STOENNER H. G. Immunologic relationships among the spotted fever group of rickettsias determined by toxin neutralization tests in mice with convalescent animal serums. J Immunol. 1960 Feb;84:171–182. [PubMed] [Google Scholar]
- BELL E. J., STOENNER H. G. Spotted fever vaccine; potency assay by direct challenge of vaccinated mice with toxin of Rickettsia rickettsii. J Immunol. 1961 Dec;87:737–746. [PubMed] [Google Scholar]
- Burgdorfer W., Sexton D. J., Gerloff R. K., Anacker R. L., Philip R. N., Thomas L. A. Rhipicephalus sanguineus: vector of a new spotted fever group rickettsia in the United States. Infect Immun. 1975 Jul;12(1):205–210. doi: 10.1128/iai.12.1.205-210.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldwell H. D., Kuo C. C., Kenny G. E. Antigenic analysis of Chlamydiae by two-dimensional immunoelectrophoresis. I. Antigenic heterogeneity between C. trachomatis and C. psittaci. J Immunol. 1975 Oct;115(4):963–968. [PubMed] [Google Scholar]
- Hoiby N., Axelsen N. H. Identification and quantitation of precipitins against Pseudomonas aeruginosa in patients with cystic fibrosis by means of crossed immunoelectrophoresis with intermediate gel. Acta Pathol Microbiol Scand B Microbiol Immunol. 1973 Jun;81(3):298–308. doi: 10.1111/j.1699-0463.1973.tb02207.x. [DOI] [PubMed] [Google Scholar]
- JACKSON E. B., SMADEL J. E. Immunization against scrub typhus. II. Preparation of lyophilized living vaccine. Am J Hyg. 1951 May;53(3):326–331. doi: 10.1093/oxfordjournals.aje.a119457. [DOI] [PubMed] [Google Scholar]
- Jepsen S., Axelsen N. H. Antigens and antibodies in Plasmodium falciparum malaria studied by immunoelectrophoretic methods. Acta Pathol Microbiol Scand C. 1980 Oct;88(5):263–270. doi: 10.1111/j.1699-0463.1980.tb00104.x. [DOI] [PubMed] [Google Scholar]
- Magnarelli L. A., Anderson J. F., Philip R. N., Burgdorfer W., Casper E. A. Endemicity of spotted fever group rickettsiae in Connecticut. Am J Trop Med Hyg. 1981 May;30(3):715–721. doi: 10.4269/ajtmh.1981.30.715. [DOI] [PubMed] [Google Scholar]
- Philip R. N., Casper E. A., Burgdorfer W., Gerloff R. K., Hughes L. E., Bell E. J. Serologic typing of rickettsiae of the spotted fever group by microimmunofluorescence. J Immunol. 1978 Nov;121(5):1961–1968. [PubMed] [Google Scholar]
- Philip R. N., Casper E. A., Ormsbee R. A., Peacock M. G., Burgdorfer W. Microimmunofluorescence test for the serological study of rocky mountain spotted fever and typhus. J Clin Microbiol. 1976 Jan;3(1):51–61. doi: 10.1128/jcm.3.1.51-61.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philip R. N., Casper E. A. Serotypes of spotted fever group rickettsiae isolated from Dermacentor andersoni (Stiles) ticks in western Montana. Am J Trop Med Hyg. 1981 Jan;30(1):230–238. doi: 10.4269/ajtmh.1981.30.230. [DOI] [PubMed] [Google Scholar]
- Philip R. N., Lane R. S., Casper E. A. Serotypes of tick-borne spotted fever group rickettsiae from western California. Am J Trop Med Hyg. 1981 May;30(3):722–727. doi: 10.4269/ajtmh.1981.30.722. [DOI] [PubMed] [Google Scholar]
- Public Health Weekly Reports for MAY 14, 1943. Public Health Rep. 1943 May 14;58(20):757–791. [PMC free article] [PubMed] [Google Scholar]
- Public Health Weekly Reports for OCTOBER 9, 1925. Public Health Rep. 1925 Oct 9;40(41):2159–2208. [PMC free article] [PubMed] [Google Scholar]
- STOENNER H. G., LACKMAN D. B., BELL E. J. Factors affecting the growth of rickettsias of the spotted fever group in fertile hens' eggs. J Infect Dis. 1962 Mar-Apr;110:121–128. doi: 10.1093/infdis/110.2.121. [DOI] [PubMed] [Google Scholar]
- Svejgaard E., Christiansen A. H. Precipitating antibodies in dermatophytosis demonstrated by crossed immunoelectrophoresis. Acta Pathol Microbiol Scand C. 1979 Feb;87C(1):23–27. [PubMed] [Google Scholar]
- Vestergaard B. F. Quantitative immunoelectrophoretic analysis of human antibodies against herpes simplex virus antigens. Infect Immun. 1979 Mar;23(3):553–558. doi: 10.1128/iai.23.3.553-558.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weeke B. A manual of quantitative immunoelectrophoresis. Methods and applications. 1. General remarks on principles, equipment, reagents and procedures. Scand J Immunol Suppl. 1973;1:15–35. doi: 10.1111/j.1365-3083.1973.tb03776.x. [DOI] [PubMed] [Google Scholar]