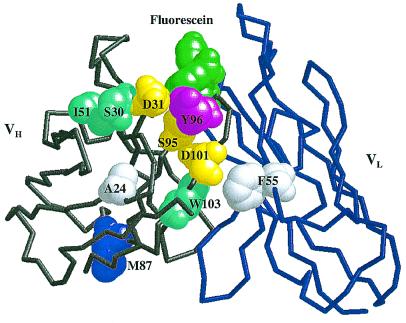
Figure 3.
Sites of consensus mutations in the 4-4-20 Fv. Backbone structure of the first 118 heavy-chain residues (gray) and the first 112 light-chain residues (blue) are shown. Fluorescein ligand (green) and mutated residues are depicted by space-filling models. Mutation sites are color-coded by distance from the binding site: first-shell residues are magenta; second-shell residues are yellow; third-shell residues are cyan; and fourth-shell residues are white. Residues in the first shell were defined as those with one or more atoms directly contacting ligand; second-shell residues were defined as those with one or more atoms directly contacting any residue in the first shell; third- and fourth-shell residues contact second- and third-shell residues, respectively. Definitions of contact were interatomic distances (in Å) equal to or less than 4.1 C-C, 3.3 O-O, 3.4 N-N and N-O, 3.8 C-N, and 3.7 C-O (48). Atomic coordinates were from the high-resolution crystal structure of Whitlow, et al. (ref. 11; PDB ID code 1FLR).