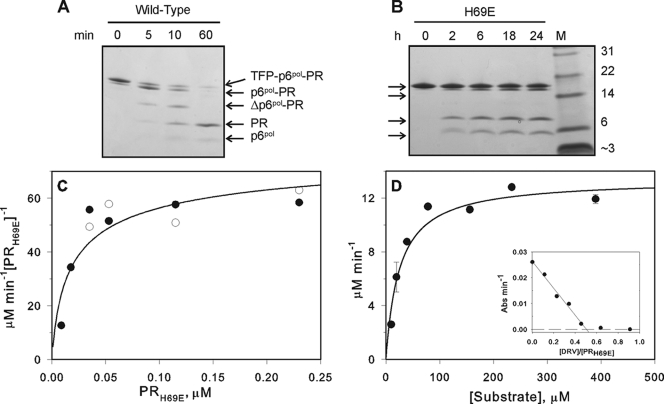
FIG. 3.
Time course of autocatalytic maturation of protease precursors in vitro and kinetic characterization of mature PRH69E. Processing reactions for the wild-type and H69E precursor proteins were conducted at final concentrations of 3.1 and 8.8 μM (A and B, respectively) in 50 mM sodium acetate, pH 5, at room temperature. Reactions were terminated as indicated (in min [A] or h [B]) and subjected to SDS-PAGE on 10 to 20% Tris-Tricine premade gels. M and Δ denote the molecular mass standards in kDa and “truncated,” respectively. The four arrows in panel B indicate TFP-p6pol-PR, p6pol-PR, PR, and p6pol, from top to bottom. The intermediate cleavage that occurs within p6pol to generate Δp6pol-PR in the wild-type precursor (A) was not observed in the H69E mutant precursor (B). (C) Initial rates of cleavage of substrate IV (0.4 mM) were measured at 28°C as a function of protein concentration (expressed as monomers) in 50 mM sodium acetate buffer, pH 5, containing 250 mM NaCl, and an apparent Kd of 30 nM was obtained by curve fitting an equation for the fraction of dimeric protease as a function of protein concentration (21) to the data. Filled and open symbols represent data from two separate experiments. (D) Kinetic parameters (Km and kcat) for hydrolysis of the chromogenic peptide, substrate IV, by 0.11 μM PRH69E (expressed as dimers) were determined under the same conditions. The inset shows a titration with the active-site-directed inhibitor DRV indicating that only ∼50% of the refolded PRH69E population possessed a functional active site.