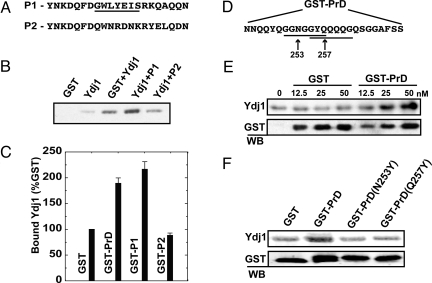
Fig. 4.
Experimental validation of Ydj1-binding motif. (A) (P1) 21-aa fragment designed with exactly 1 consensus-binding sequence (underlined). (P2) 21-aa scrambled peptide with no consensus-binding sequence. (B) The protein complex was isolated with glutathione-conjugated beads and resolved by SDS/PAGE. (C) P1 showed a 2-fold increase in binding compared with GST alone, whereas signals from binding to P2 remained at the background. (D) A 25-aa fragment from the Rnq1 prion domain (amino acids 245–269) contains 2 overlapping Ydj1-binding motifs (underlined). To demonstrate direct binding, this fragment was fused to GST and incubated with purified Ydj1. Similar experiments were conducted to demonstrate binding of the PrD peptide to Ydj1p. (E) The GST-PrD fusion was titrated with 50 nM Ydj1 to determine the concentration at which binding between GST-PrD and Ydj1 is saturated. Data points were generated by quantifying bound Ydj1 after Western immunoblotting and normalizing to background (no GST protein). (F) Point mutations were made in the third position of 2 putative Ydj1-binding motifs in GST-PrD. Ydj1 was incubated with GST alone, GST-PrD, or GST-PrD with these point-mutants at a 1:2 ratio to determine binding affinity.