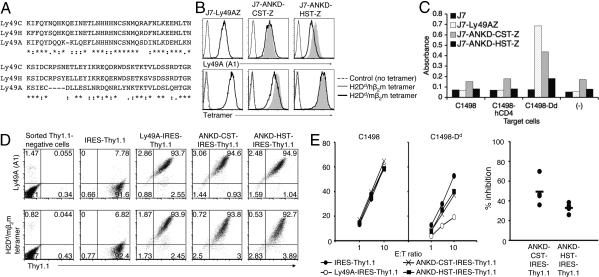
Fig. 4.
Stalk-chimeric Ly49A receptors effectively bind ligand, but show functional discrepancy in signaling. (A) Stalk sequences of Ly49A, Ly49C, and Ly49H. Identical and conserved residues are marked with asterisks and colons, respectively. (B) Surface expression of stalk-chimeric receptors. Ly49AZ, ANKD-CST-Z, and ANKD-HST-Z on J7 reporter cells were stained with anti-Ly49A mAb (A1) and H2Dd/mβ2m tetramers. Gray shades represent the overlaid histogram from Ly49AZ. (C) Trans interaction reporter assays were performed with indicated target and reporter cells. (D) FACS profiles of sorted Thy1.1-positive LAK cells. LAK cells were prepared from B6 mice treated with A1 mAb, which depleted 90–95% of endogenous Ly49A-positive cells when assessed by A1 staining with day 9 LAK cells. LAK cells were infected with lentiviruses encoding wild-type Ly49A and mutant receptors with IRES-Thy1.1 on day 3 and sorted for Thy1.1 expression on day 6. Sorted cells were stained with anti-Ly49A mAb (A1) and H2Dd/mβ2m tetramers on day 9. No binding was observed with control H2Dd/hβ2m tetramers. (E) Killing assays were performed with indicated target cells and sorted Thy1.1-positive LAK cells infected with lenti-virus encoding IRES-Thy1.1 (Left). Thy1.1-negative LAK cells from each transductant killed C1498 and C1498-Dd target cells similarly. Relative inhibitory capabilities of mutant receptors compared with the wild-type Ly49A receptor from 4 experiments are shown (Right). Thy1.1-negative LAK cells from each transductant showed similar killing to C1498 and C1498-Dd target cells. No difference was observed in killing of C1498 target cells between Thy1.1-negative and -positive LAK cells. No statistically significant difference was observed between ANKD-HST and ANKD-CST in the relative inhibitory capabilities in the combined data from 4 separate experiments.