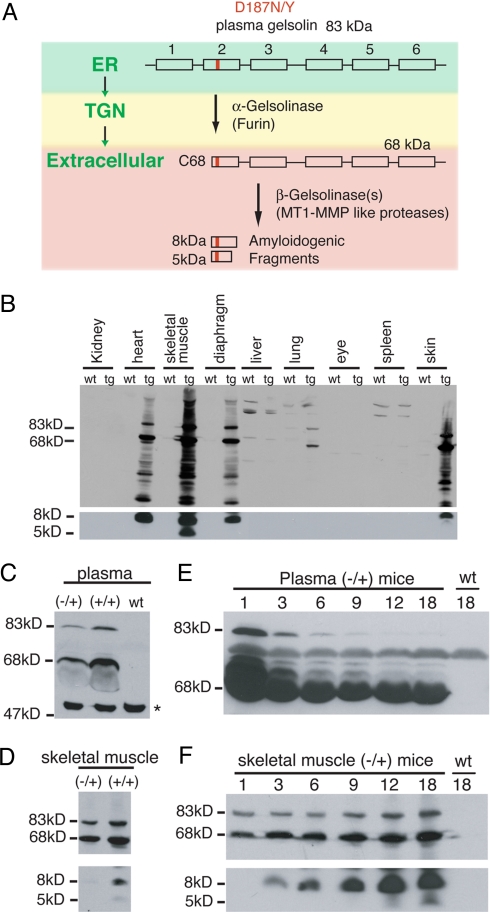
Fig. 1.
Proteolytic fragments of D187N plasma gelsolin are present in transgenic mice. (A) Scheme of D187N/Y (mutation is highlighted in red) plasma gelsolin proteolytic cascade and cleavage products detected in FAF patients. The 6 domains of gelsolin are depicted by rectangles. The subcellular or extracellular location of each event is indicated in green. A proportion of D187N/Y is cleaved by furin in the trans-Golgi network (TGN) to generate a 68-kDa protein (C68) that is secreted. C68 is further processed extracellularly by MT1-MMP-like proteases, to form the 8- and 5-kDa amyloidogenic fragments that are deposited in FAF patients. (B) Western blot of tissues from 18-month D187N (−/+) gelsolin mice and wild-type siblings. In addition to full-length (83-kDa) plasma gelsolin, many proteolytic fragments, including the 68- and 8-kDa peptides, were detected in cardiac and striated muscle tissue from D187N (−/+) transgenic (tg) mice but not from their wild-type (wt) siblings. No or very low expression levels of D187N gelsolin were detected in nonmuscle tissues. (C and D) Homozygous D187N (+/+) mice have approximately double the level of human D187N plasma gelsolin in plasma (C) and skeletal muscle (D) compared with the hemizygous D187N (−/+) mice. The asterisk (∗) indicates a cross-reacting protein that is also found in wild-type animals that do not express human D187N gelsolin. (E) The amount of human D187N plasma gelsolin in plasma decreases with the age of the mice (in months). (F) The amount of human D187N plasma gelsolin in skeletal muscle increases as the age of the mouse (in months) increases. No expression of human D187N gelsolin is detected in the wild-type animal (wt).