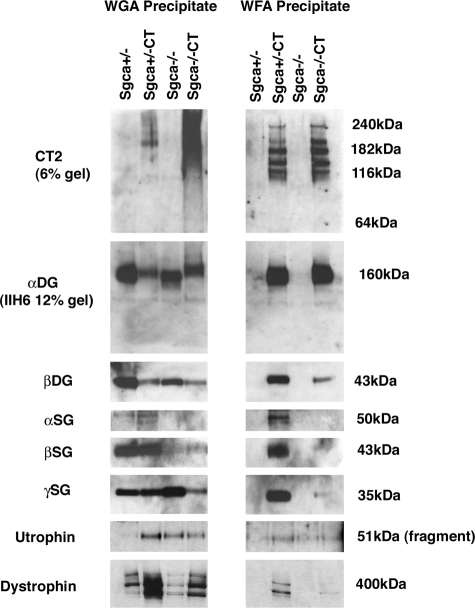
Figure 6.
Loss of α sarcoglycan does not affect glycosylation of α dystroglycan with the CT carbohydrate in Galgt2 transgenic mice. Gastrocnemius muscle was solubilized in non-ionic detergent and precipitated with Wheat germ agglutinin (WGA), a control lectin known to bind α dystroglycan, and Wisteria floribunda agglutinin, a βGalNAc-binding lectin that binds the CT-glycosylated form of α dystroglycan. α dystoglycan was glycosylated by Galgt2 such that it could be precipitated by WFA equally well in Sgca+/−CT and Sgca−/−CT muscle. In non-CT muscle, WGA bound as much α dystroglycan as WFA did in CT muscle. Precipitates resolved on a low percentage gel (6%) and blotted with CT2 show equivalent glycoforms of α dystroglycan precipitated by WFA in Sgca+/−CT and Sgca−/−CT muscle. β dystroglycan was co-precipitated with α dystroglycan in all muscles, but was relatively reduced in WFA precipitates from Sgca−/−CT, as were β and γ sarcoglycan, due to their reduced overall expression in these lysates (see Figure 7). Full-length dystrophin (427 kDa) and a utrophin protein fragment (51 kDa) were also enriched in CT muscles. Data are representative of three experiments with similar results.