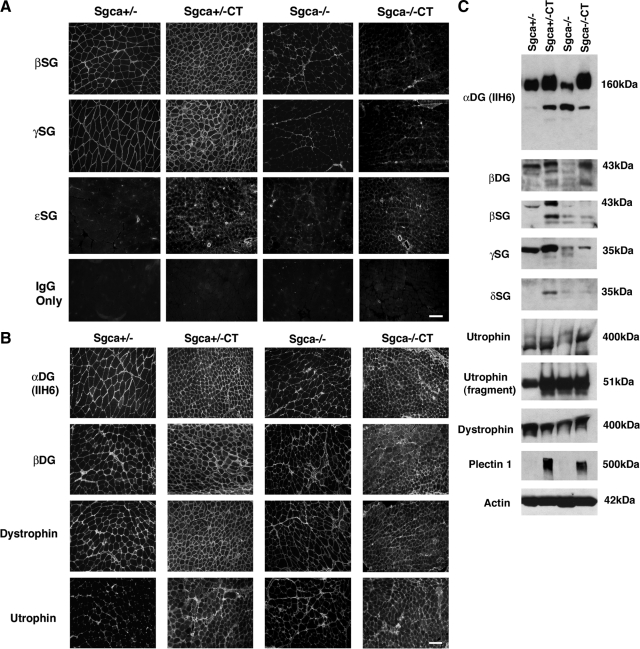
Figure 7.
β, γ, δ, and ε sarcoglycan are not overexpressed in Galgt2 transgenic Sgca−/− muscle. A: Immunostaining of gastrocnemius muscle in Galgt2 transgenic (CT) and non-transgenic Sgca+/− and Sgca−/− mice. β, γ, and ε sarcoglycan were not increased in expression along myofibers in Sgca−/−CT muscle, while (B) immunostaining of α dystroglycan, β dystroglycan, dystrophin, and utrophin was high in Sgca+/−CT and Sgca−/−CT muscle. Scale bar =100 μm (A and B). C: 40 μg of SDS whole muscle lysate from gastrocnemius is loaded per lane. β, γ and δ sarcoglycan protein levels were not increased in Sgca−/−CT muscle as they were in Sgca+/−CT muscle. Utrophin, α dystroglycan, and plectin 1, by contrast, were similarly increased in both Sgca−/−CT and Sgca+/−CT muscle. Actin is shown as a control for protein loading and transfer.